Minard-Colin et al recently reported for the European Intergroup for Childhood Non-Hodgkin Lymphoma/Children’s Oncology Group (EICNHL/COG) a significant improvement in event-free survival among children and adolescents (aged 6 months to 18 years) with high-risk mature B-cell non-Hodgkin lymphoma (B-NHL) following the addition of six doses of rituximab, administered by dose-dense infusion, to two standard French-American-British (FAB) chemotherapy backbones compared with FAB chemotherapy backbones alone.1 The study is reviewed in this issue of The ASCO Post.

Mitchell S. Cairo, MD
High-risk disease was defined as patients with stage III disease with lactate dehydrogenase (LDH) levels at least two times the upper limit of normal (ULN) or stage IV disease including patients with bone marrow and/or central nervous system (CNS) involvement, as defined previously by the FAB/LMB96 trial.1-4 The current study was based on our prior phase II trial of the investigation of dose-dense rituximab administration combined with the same two FAB/LMB96 chemotherapy backbones in the same population of patients. The results of the current trial (Inter-B-NHL 2010) report event-free survival and other expected results in the experimental arm similar to those shown in our previous phase II trial.5,6
The authors of the Inter-B-NHL 2010 trial did not discuss the importance of the dose-dense administration of rituximab, although this may be of critical importance in patients with bulky and/or disseminated disease, such as occurs in children and adolescents with high-risk mature Burkitt lymphoma/leukemia, as described in adults with diffuse large B-cell lymphoma (DLBCL) by Pfreundschuh et al.7,8 Our group previously reported that two doses of rituximab administered 48 hours apart (375 mg/m2/dose; dose-dense) during both chemoimmunotherapy induction cycles resulted in higher peak and trough levels of rituximab, was well tolerated, and appeared to significantly increase event-free survival compared with historical control trials that evaluated FAB/LMB96 chemotherapy alone.5-7
Evolving Treatment
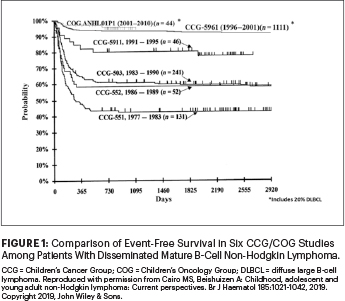
In the past 40 years, there has been dramatic improvement in the prognosis (event-free survival) in children and adolescents with disseminated (high-risk) mature B-NHL. As illustrated in Figure 1, the Children’s Cancer Group (CCG) and subsequently COG demonstrated a steady increase in event-free survival in this group of patients while reducing the length of therapy from 2.5 years to 3 to 6 months, eliminating both local and CNS radiotherapy, reducing total doses of cyclophosphamide and doxorubicin, and more recently incorporating targeted monoclonal rituximab immunotherapy.2-6,9-14
The results of this international study, confirming our COG pilot studies, firmly establish that the addition of dose-dense rituximab to FAB/LMB96 chemotherapy backbones represents the “standard” approach in children and adolescents with high-risk mature B-NHL. We eagerly await the results of additional studies comparing one vs six doses of rituximab in combination with the Berlin-Frankfurt-Munich (BFM) chemotherapy backbone (ClinicalTrials.gov identifier NCT03200671).15
Safety Profile Considerations
Comparing toxicities among the original FAB/LMB96 studies2-4 (chemotherapy alone) vs the COG ANHL01P1 pilot study5,6 (dose-dense rituximab with best chemotherapy backbones of FAB/LMB96) vs the Inter-B-NHL 2010 study, there appears to be a persistently higher risk of grade III or IV mucositis (stomatitis) and infection rates in the Inter-B-NHL 2010 trial. However, most concerning is the 75% and 80% risk of mucositis in the chemotherapy-alone arm and rituximab-plus-chemotherapy arm in the Inter-B-NHL 2010 trial compared with the results of the rituximab-plus-FAB/LMB96 pilot trial, which showed 11% and 9% risks of grade III or IV mucositis in group B and group C patients, respectively (Table 1).
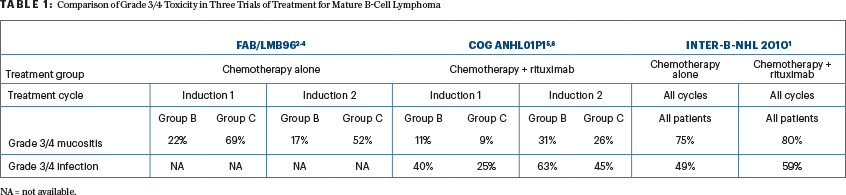
In the original FAB/LMB96 study, doxorubicin was initially administered as a continuous 48-hour infusion, which resulted in grade III or IV mucositis rates of 92% and 74%, respectively, in induction cycles 1 and 2. The study was amended to reduce the doxorubicin infusion to 6 hours, which resulted in a significant reduction in grade III or IV mucositis in each induction cycle, to 79% and 67%, respectively (P < .05). The COG ANHL01P1 pilot study further reduced the doxorubicin infusion time to either intravenous push or a 30-minute infusion, and the rate of grade III or IV mucositis was dramatically decreased to 11% and 9% (group B) and 31% and 26% (group C) in induction cycles 1 and 2, respectively (Table 1).2-6
It is unclear why the risk of grade III or IV mucositis was significantly increased in the Inter-B-NHL 2010 study (back to an incidence of 75% to 80%) despite utilizing the shorter infusion of doxorubicin (Table 1). This higher rate of mucositis will likely contribute to both prolonged hospitalization and grade III or IV infections.
Remaining Issues
Future investigations in childhood and adolescent mature B-NHL should include assessments of the role of rituximab in favorable-risk patients (stage I/II and low LDH stage III), optimal therapeutic approaches in patients with relapsed or refractory disease, and novel approaches for patients with high-risk and/or unusual subtypes of mature B-NHL. We have recently demonstrated the safe and effective reduction of doxorubicin to a total dose of 50 mg/m2 with the addition of six doses of dose-dense rituximab to the group B FAB/LMB96 backbone and demonstrated a 100% event-free survival in 25 consecutive patients.16
Children and adolescents with relapsed or refractory disease also have a dismal prognosis due in large part to chemotherapy resistance, and novel approaches are urgently needed in this group of patients.17,18 In children and adolescents with relapsed or refractory disease, our group previously demonstrated numerous other surface targets in mature B-NHL, including CD22, CD19, CD79, among others, and have investigated in both clinical and preclinical studies novel humoral and cellular immunotherapy approaches in an attempt to circumvent chemotherapy-resistant disease.19-21
Most recently, we reported the safety and pharmacokinetics of adding the Bruton’s tyrosine kinase inhibitor ibrutinib to two common chemotherapy reinduction chemotherapy backbones.22 Furthermore, rituximab-containing FAB/LMB96 therapy is not only difficult to administer in lower socioeconomic countries, but the toxicities are difficult to manage without highly intensified supportive care, and novel, strategic approaches are required in this unique population as well.23
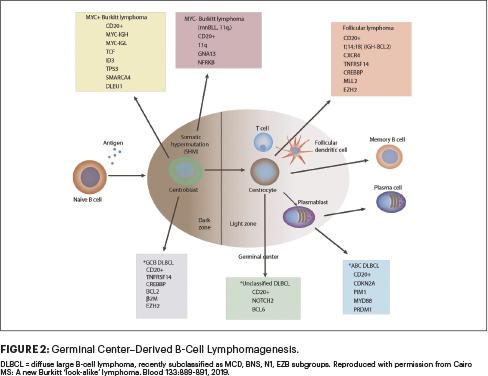
Additionally, novel approaches are required for patients with rarer subtypes of mature B-NHL, including those with primary mediastinal B-cell lymphoma, activated B-cell DLBCL, MYC-negative Burkitt lymphoma, among others within germinal cell–derived B-cell lymphoma populations (Figure 2).24-26 Additionally, the impact of minimal residual disease and high-risk cytogenetics on event-free survival is not addressed in the Inter-B-NHL 2010 trial and will need to be addressed in the future.27-29
Conclusions
Although the results of the Inter-B-NHL 2010 study demonstrated a significant improvement in event-free survival in children and adolescents with high-risk mature B-NHL, it has taken more than 18 years since the landmark report of Coiffier et al showed a significant benefit in event-free survival with rituximab plus CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) vs CHOP alone in elderly patients with DLBCL. It is imperative that we form collaborations within academic communities, pediatric oncology cooperative groups, governmental cancer programs, the pharmaceutical industry, and governmental regulatory agencies to identify a more accelerated platform for drug development in children and adolescents. Our goal should be to more rapidly complete the required phase III trials, so children and adolescents “don’t have to wait another 18 years” before a new standard of care is established.9,30
Dr. Cairo is Director of the Children and Adolescent Cancer and Blood Diseases Center, at Westchester Medical Center Cancer Center and Professor of Pediatrics, Medicine, Pathology, Microbiology & Immunology, and Cell Biology & Anatomy at New York Medical College in Valhalla, New York.
DISCLOSURE: Dr. Cairo reported no conflicts of interest.
REFERENCES
1. Minard-Colin V, et al: Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med 382:2207-2219, 2020.
2. Cairo MS, et al: Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 109:2736-2743, 2007.
3. Cairo MS, et al: Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (>/= 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma. J Clin Oncol 30:387-393, 2012.
4. Patte C, et al: Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents. Blood 109:2773-2780, 2007.
5. Goldman S, et al: Rituximab and FAB/LMB 96 chemotherapy in children with stage III/IV B-cell non-Hodgkin lymphoma. Leukemia 27:1174-1177, 2013.
6. Goldman S, et al: Rituximab with chemotherapy in children and adolescents with central nervous system and/or bone marrow-positive Burkitt lymphoma/leukaemia. Br J Haematol 167:394-401, 2014.
7. Barth MJ, et al: Rituximab pharmacokinetics in children and adolescents with de novo intermediate and advanced mature B-cell lymphoma/leukaemia. Br J Haematol 162:678-683, 2013.
8. Pfreundschuh M, et al: CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma. Lancet Oncol 7:379-391, 2006.
9. Cairo MS: Rituximab in the treatment of childhood mature B-cell lymphoma: ‘Where do we go from here.’ Br J Haematol 185:1017-1020, 2019.
10. Cairo MS, Beishuizen A: Childhood, adolescent and young adult non-Hodgkin lymphoma: Current perspectives. Br J Haematol 185:1021-1042, 2019.
11. Cairo MS, et al: Long-term follow-up of short intensive multiagent chemotherapy without high-dose methotrexate (‘Orange’) in children with advanced non-lymphoblastic non-Hodgkin’s lymphoma. Leukemia 16:594-600, 2002.
12. Cairo MS, Pinkerton R: Childhood, adolescent and young adult non-Hodgkin lymphoma: State of the science. Br J Haematol 173:507-530, 2016.
13. Frazer JK, et al: Excellent outcomes in children and adolescents with CNS(+) Burkitt lymphoma or other mature B-NHL using only intrathecal and systemic chemoimmunotherapy. Br J Haematol 185:374-377, 2019.
14. Miles RR, et al: Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br J Haematol 156:730-743, 2012.
15. Meinhardt A, et al: Phase II window study on rituximab in newly diagnosed pediatric mature B-cell non-Hodgkin’s lymphoma and Burkitt leukemia. J Clin Oncol 28:3115-3121, 2010.
16. Goldman S, et al: Reduced burden of oncologic therapy in children, adolescents and young adults with good risk (GR) CD20+ mature B-cell lymphoma. Br J Haematol 182:11 (Abstract 10), 2018.
17. Cairo M, et al: Overall survival of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after treatment with FAB/LMB 96. Br J Haematol 182:859-869, 2018.
18. Jourdain A, et al: Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective ‘Lymphomes Malins B’ protocols. Haematologica 100:810-817, 2015.
19. Awasthi A, et al: Obinutuzumab compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20(+) rituximab-sensitive-resistant Burkitt lymphoma and precursor B-acute lymphoblastic leukaemia. Br J Haematol 171:763-775, 2015.
20. Chu Y, et al: Advances in cellular and humoral immunotherapy. Br J Haematol 185:1055-1070, 2019.
21. Miles RR, et al: Immunophenotypic identification of possible therapeutic targets in paediatric non-Hodgkin lymphomas. Br J Haematol 138:506-512, 2007.
22. Burke GAA, et al: Ibrutinib plus CIT for R/R mature B-NHL in children (SPARKLE trial). Leukemia. February 18, 2020 (early release online).
23. Ngoma T, et al: Treatment of Burkitt lymphoma in equatorial Africa using a simple three-drug combination followed by a salvage regimen for patients with persistent or recurrent disease. Br J Haematol 158:749-762, 2012.
24. Cairo MS: A new Burkitt ‘look-alike’ lymphoma. Blood 133:889-891, 2019.
25. Gerrard M, et al: Outcome and pathologic classification of children and adolescents with mediastinal large B-cell lymphoma treated with FAB/LMB96 mature B-NHL therapy. Blood 121:278-285, 2013.
26. Wagener R, et al: The mutational landscape of Burkitt-like lymphoma with 11q aberration is distinct from that of Burkitt lymphoma. Blood 133:962-966, 2019.
27. Poirel HA, et al: Specific cytogenetic abnormalities are associated with a significantly inferior outcome in children and adolescents with mature B-cell non-Hodgkin’s lymphoma. Leukemia 23:323-331, 2009.
28. Shiramizu B, et al: Minimal disease assessment in the treatment of children and adolescents with intermediate-risk (stage III/IV) B-cell non-Hodgkin lymphoma. Br J Haematol 153:758-763, 2011.
29. Shiramizu B, et al: Impact of persistent minimal residual disease post-consolidation therapy in children and adolescents with advanced Burkitt leukaemia. Br J Haematol 170:367-371, 2015.
30. Coiffier B, et al: CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med.

