
Nirali Patel, MD, Assistant Professor of Pathology and Laboratory Medicine at the University of North Carolina (UNC) Lineberger Comprehensive Cancer Center, and Norman E. Sharpless, MD, Director of the UNC Lineberger Comprehensive Cancer Center, review the clinical analysis Watson for Genomics generated for a patient with endometrial cancer. (Editor’s note: Earlier this month, Dr. Sharpless was appointed by President Donald Trump to serve as Director of the National Cancer Institute.)
After undergoing nearly 5 years of intensive medical training, IBM’s Watson for Oncology cognitive computing system is starting to make good on its promise to accelerate personalized care for patients with cancer. The system has been trained by oncologists at Memorial Sloan Kettering Cancer Center (MSK) in New York to learn key data associated with a patient’s cancer—including results from blood tests; pathology and imaging reports detailing the type, size, and location of the tumor; and the presence of genetic mutations—and then comb through huge amounts of medical literature to prioritize evidence-based treatment for that specific patient (Figures 1 and 2). Now, studies are showing that Watson is achieving a high degree of concordance with physician recommendations.
Using natural language processing software to understand written language and probabilistic algorithms, Watson is able to read and understand millions of pages of text to zero in on information relevant to clinicians’ queries and generate multiple possibilities for use in making treatment decisions. Initially trained in lung, breast, and colorectal cancers, early studies by Memorial Sloan Kettering show that Watson had greater than 90% agreement with MSK treatment recommendations for patients with stage I to III breast cancer1 and matched MSK physician recommendations 50% of the time for patients with lung cancer. In 16 cases of advanced lung cancer, 88% of the administered regimens were returned as either “Watson recommended” or “Watson recommended for consideration.”2
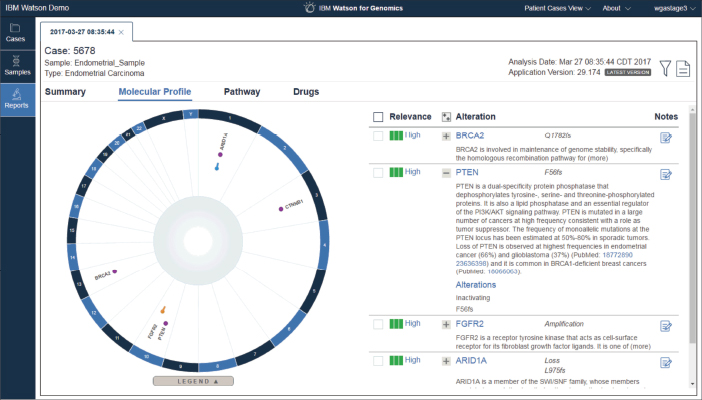
Figure 1. This screenshot of Watson for Genomics shows the molecular profile of an endometrial carcinoma, including genetic alterations that are known to be related to cancer. The alterations are classified as pathogenic, likely pathogenic, or variants of unknown significance. The interface also displays the evidence extracted from the medical literature to support classification. Screenshot courtesy of IBM.
More recently, a large study of 638 patients with breast cancer by researchers at Manipal Comprehensive Cancer Center in Bengaluru, India, found that, overall, 90% of Watson recommendations or recommendations for consideration were concordant with treatment recommendations from the cancer center’s tumor board. When the researchers compared the amount of time it took “manually” and using Watson to capture and analyze data to generate recommendations, they found that the manual approach took an average of 20 minutes, which was reduced to 12 minutes once physicians gained more familiarity with the cases. It took just 40 seconds for Watson to analyze the data and give a treatment recommendation.3 In addition, at the 2017 ASCO Annual Meeting, IBM presented data on five study abstracts showing that, overall, Watson’s treatment recommendations were concordant with tumor board treatment recommendations in up to 96% of patient cases examined and that Watson reduced the amount of screening time of patient charts for clinical trial eligibility by 78%.4–8
“What I’ve learned so far is that whatever we thought about the potential of this cognitive computing system as a physician decision support tool is true,” said Mark G. Kris, MD, Lead Physician of the Memorial Sloan Kettering–IBM Watson Collaboration.

“All of us involved in training Watson … are absolutely convinced that this technology will become an indispensable part of a doctor’s armamentarium to care for patients.”— Mark G. Kris, MD
Tweet this quote
“And the need for this type of clinical support tool has only grown since we started training Watson 5 years ago. All of us involved in training Watson, which is equivalent to how we would train a young doctor, are absolutely convinced that this technology will become an indispensable part of a doctor’s armamentarium to care for patients, and the need for this type of device at the point of care will only grow.”
Dr. Kris and his colleagues are now teaching Watson the molecular characteristics of cancers, and training in gastric, cervical, ovarian, and prostate cancers is underway.
Quick Learner
What once took Watson 6 to 9 months to learn about a specific cancer now takes about 30 days, according to John Kelly, PhD, Senior Vice President of Cognitive Solutions and IBM Research. The software is being tested in about 30 cancer centers and dozens of research institutions in the United States and Asia.
Although Watson is still in the testing phase in most U.S. cancer centers, the cloud-based system was recently implemented at Jupiter Medical Center in Florida in breast, lung, colorectal, gastric, cervical, and ovarian cancers. In addition, it is being used in the clinical setting in several hospitals in Thailand, India, Korea, and Slovakia and is being adopted in hospital systems throughout China.
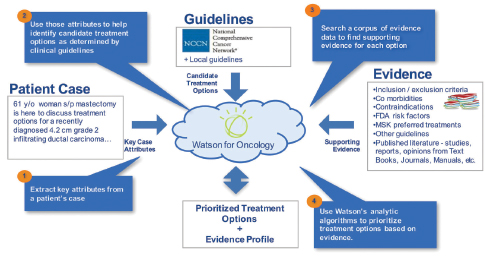
Figure 2. To provide physicians with evidence-based treatment recommendations, Watson for Oncology extracts key attributes from a patient record, identifies various treatment recommendations, and ranks them according to the level of supporting evidence curated by Memorial Sloan Kettering Cancer Center. Illustration courtesy of IBM.
“Our strategy was to introduce Watson to the major cancer centers in the United States first, so it could get the training and be adopted into U.S. health-care systems, and then take the technology global, where the ratio of oncologists to patients with cancer is far inferior to the United States,” said Dr. Kelly.
Although there are no published studies yet on how Watson’s advice is improving physician decision-making and impacting patient outcome, there is a sense that the supercomputer is making a positive difference in oncology care. “I can’t give you a clinical trial result yet,” said Dr. Kris. “But there is a perception that Watson is improving patient care and that it is putting together the most critical information in a way that can be shared and used. There is agreement among oncologists that not only does Watson provide information that helps the physician and the care team in their decision-making, it also tells you what critical pieces of information are missing, what tests need to be ordered, and what the evidence is to support treatment choices.”
Multiple Applications
In addition to Watson for Oncology, IBM recently launched several other oncology-related solutions aimed at accelerating personalized patient care. They include Watson for Genomics (Figure 1), Watson Genomics from Quest Diagnostics (a Watson-powered genomic-sequencing service), and IBM Watson for Clinical Trial Matching, which identifies potential clinical trial options for patients (see Figure 3).
In January, IBM announced that it was partnering with DNA-sequencing equipment maker Illumina to integrate Watson for Genomics into the company’s tumor-sequencing process to help standardize and simplify genomic data interpretation. The aim is to, in a matter of minutes, allow researchers to read the genetic alteration files produced by Illumina’s tumor assay; cull medical guidelines, literature, and clinical trials compendia to provide information for each genomic alteration; and produce a report—a process that usually takes more than a week to complete. To guarantee accurate results, Watson is programmed to grade scientific literature based on the quality of available evidence, the same standard used by human scientists.

“Once you leverage these tools for discovery around drug combinations and have the ability to stratify patient populations for clinical trials, it should improve the efficiency of clinical trials, increase the speed at which trials are completed, and, hopefully, result in many more effective therapies for patients.”— John Kelly, PhD
Tweet this quote
Watson’s cognitive computing ability has also been tapped to speed research in immunotherapy. This past December, Pfizer teamed up with IBM Watson Health to use the technology company’s latest cognitive computing tool, Watson for Drug Discovery, to help researchers identify new drug targets, potential combination therapies for study, and patients for clinical trials. Although studies show that researchers are only able to read between 200 and 300 research articles in a year to try to stay current on the latest medical results,9 Watson’s capacity to decipher huge amounts of data and interpret clinical information seemingly knows no bounds. Watson’s drug discovery platform has already been fed 25 million Medline abstracts, more than 1 million medical journal articles, and 4 million patents, and each month, Watson ingests about 10,000 new scientific articles and data on 100 new clinical trials to keep up-do-date on new findings.
“I always felt that the most important application for Watson would be in the area of discovery, new science, new treatments, and certainly new pharmaceuticals,” said Dr. Kelly. “I’m not trying to diminish the importance of Watson as a clinical support aid, but using this technology to crawl through massive amounts of research data will produce amazing results. Once you leverage these tools for discovery around drug combinations and have the ability to stratify patient populations for clinical trials, it should improve the efficiency of clinical trials, increase the speed at which trials are completed, and, hopefully, result in many more effective therapies for patients.”
Zeroing in on Genetic Mutations
One of the early adopters of the Watson for Genomics platform is the University of North Carolina (UNC) Lineberger Comprehensive Cancer Center, Chapel Hill, which began using Watson in 2015 as a standard part of its molecular tumor board process. The cancer center employed Watson to analyze data from recent clinical trials and provide aggregated information based on the quality of available scientific evidence. Tumor board members could then use that knowledge to better inform patient care.

“Watson’s gene identification provided a rationale to offer another therapy that was perhaps less toxic than a traditional chemotherapy or apply for a compassionate use exemption because the patient had no other feasible options.”— Nirali Patel, MD
Tweet this quote
According to Nirali Patel, MD, Assistant Professor of Pathology and Laboratory Medicine at the University of North Carolina, each week, the molecular tumor board meets to discuss about 20 patient cases, many of which have exhausted standard-of-care therapies, to look for potential new treatments based on the unique DNA mutations that are driving each patient’s tumor. In a study UNC conducted with 1,000 actual patient cases to compare Watson’s genomic analysis with the analysis of the center’s tumor board, the investigators found that Watson identified the same potential therapies as the tumor board 99% of the time. But what was more extraordinary, in about 300 patients, Watson found clinically actionable information that the tumor board had not identified.
“We had a list of about 150 genes that we thought were actionable, and those genes ended up on Watson’s list as well,” said Dr. Patel. “In addition, Watson found 7 or 8 actionable genes that our curators had not managed to find literature on or for which they had not realized there was a clinical trial open. The additional information allowed us to say, ‘On a second review, these genes should be incorporated into our clinical reporting because we can act on them.'"

Figure 3. In a demonstration, Watson for Clinical Trial Matching identifies a phase II trial that may be appropriate for a 42-year-old patient with breast cancer. By automatically comparing enrollment criteria with structured and unstructured patient data, the AI technology can reduce clinical trial screening time by 78%, according to data presented at the 2017 ASCO Annual Meeting. Screenshot courtesy of IBM.
According to Dr. Patel, some of those 300 patients who had previously exhausted their treatment options were now presented with another opportunity for care. “Watson’s gene identification provided a rationale to offer another therapy that was perhaps less toxic than a traditional chemotherapy or apply for a compassionate use exemption because the patient had no other feasible options,” said Dr. Patel. The UNC study has been submitted to a peer-reviewed journal for publication and is currently under review.
Watson for Genomics now includes annotations for all genetic variants in more than 460 cancer-associated genes in any tumor type. “Over time, Watson will get smarter and not just look at a genomic mutation and analyze what it means, but also consider other medical problems the patient has and further refine its output on what might be a more pertinent treatment for that patient, and help us improve patient care,” said Paul Sabbatini, MD, Deputy Physician-in-Chief for Clinical Research at Memorial Sloan Kettering Cancer Center.

“Over time, Watson will get smarter and not just look at a genomic mutation and analyze what it means, but also consider other medical problems the patient has and further refine its output on what might be a more pertinent treatment for that patient.”— Paul Sabbatini, MD
Tweet this quote
Providing Patient-Centered Care
Ultimately, said Dr. Kris, the benefit of Watson as a clinical support tool is in its ability to render a complete assessment of each patient’s cancer and offer physicians a complete care plan. “The first use of Watson is to provide a platform to show the different treatment choices for a specific patient and use that information in the treatment decision-making process,” said Dr. Kris. “What I’m really excited about is, we’ve built into Watson the ability to create a total care plan for patients with breast cancer that includes not just the treatment the oncologist is planning today, but what may be needed in the future, such as surgery, radiation therapy, or hormonal therapy, so the patient knows what to expect in both the short and long term.”
He concluded, “With each passing day, the integration of cognitive computing into our medical health record system just keeps growing. Watson is going to become part of the care for every patient.” ■
DISCLOSURE: Dr. Kelly is IBM Senior Vice President, Cognitive Solutions and IBM Research, and his work is focused on IBM’s investments in the information technology market. Dr Kris reports receiving personal fees from Genentech/Roche, AstraZeneca, and ARIAD. Dr. Patel reports no conflicts of interest. Dr. Sabbatini has received research funding from Janssen Oncology and Bristol-Myers Squibb.
REFERENCES
9. Van Noorden R: Scientists may be reaching a peak in reading habits. Nature (News). February 2014. Available at www.nature.com/news/scientists-may-be-reaching-a-peak-in-reading-habits-1.14658. Accessed May 2, 2017.

