In patients with metastatic pancreatic cancer and germline mutations in BRCA1 or BRCA2, maintenance therapy with olaparib doubled the time to disease progression and the proportion of patients who were progression-free at 2 years, in the phase III POLO trial.1

Hedy Lee Kindler, MD
“Maintenance olaparib provided a statistically significant and clinically meaningful 47% improvement in progression-free survival,” said Hedy Lee Kindler, MD, Professor of Medicine at the University of Chicago, who presented the POLO trial findings at the 2019 ASCO Annual Meeting’s Plenary Session. Patients receiving maintenance therapy after stable or responding disease to initial platinum-based chemotherapy had a median progression-free survival of 7.4 months, compared with 3.8 months for those on placebo (hazard ratio [HR] = 0.53; P = .0038).
The findings were concurrently published in The New England Journal of Medicine.2 The inhibitor of poly (ADP-ribose) polymerase (PARP) is approved by the U.S. Food and Drug Administration in the treatment of patients with ovarian and breast cancers who have BRCA mutations.
Oncologists applauded these study findings. At a press briefing, ASCO spokesperson Suzanne Cole, MD, Director of the University Hospital Simmons Cancer Clinic at the UT Southwestern Medical Center at Richardson/Plano, commented, “These results are practice-changing for our patients. I can’t wait to go back to the clinic and look for BRCA mutations in my patients.”

Suzanne Cole, MD
Dr. Cole continued: “Now that we have a targeted medicine that can benefit patients with BRCA mutations, it is our duty to search for this mutation to identify those who will benefit from a treatment that could extend their life.”
Rationale Behind Novel Therapy
As Dr. Kindler noted, metastatic pancreatic cancer is a “dismal disease,” with a median progression-free survival of about 6 months and a median overall survival of 8 to 12 months with the current standard-of-care chemotherapy. Fewer than half of patients are able to proceed to second-line therapy, and, until now, there has been no effective targeted therapy. Maintenance treatments aim to delay disease progression following chemotherapy without compromising quality of life.
Some 4% to 7% of patients with metastatic pancreatic cancer harbor a germline BRCA mutation. In other tumor types, these BRCA-deficient tumors have derived a benefit from platinum-based chemotherapy and from PARP inhibitors. Olaparib works by trapping PARP at sites of DNA single-strand breaks, causing an accumulation of DNA damage and tumor-cell death.
“Our results are the first from a phase III trial to validate a targeted treatment in a biomarker-selected population of patients with pancreatic cancer,” Dr. Kindler noted.
“Our results are the first from a phase III trial to validate a targeted treatment in a biomarker-selected population of patients with pancreatic cancer.”— Hedy Lee Kindler, MD
Tweet this quote
POLO Details
The phase III POLO trial was conducted at 119 sites in 12 countries. Of 3,315 patients with pancreatic cancer screened, 247, or 7.5%, had germline BRCA mutations, and 154 were enrolled after not experiencing disease progression during 16 weeks or more of platinum-based chemotherapy. The investigators randomly assigned 92 patients to treatment with olaparib at 300 mg twice daily and 62 patients to the placebo arm. Maintenance was initiated 4 to 8 weeks after the last dose of chemotherapy and continued until radiologic disease progression by investigator assessment.
Improvements in All Key Outcomes
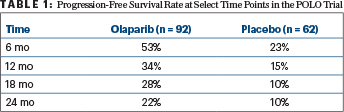
The primary endpoint was progression-free survival by blinded independent central review. The progression-free survival was 7.4 months with maintenance olaparib, compared with 3.8 months with placebo (hazard ratio [HR] = 0.53; P = .0038). From 6 months onward, more than twice the proportion of olaparib-treated patients were progression-free (Table 1). Time to second disease progression was improved by 24% as well, “which may indicate the durability of treatment benefit beyond disease progression,” she suggested.
“What was truly remarkable was that the median duration of response in patients with metastatic pancreatic cancer was more than 2 years,” Dr. Kindler emphasized. She predicted that this could signal a change in the disease trajectory of a pancreatic cancer subset.
The objective response by blinded independent central review was 23.1% with olaparib and 11.5% with placebo. Two olaparib-treated patients had complete responses, and both were ongoing at the time of data cutoff. The planned interim analysis of overall survival (at 46% maturity) demonstrated no difference in survival, with median survival times of approximately 18 months.
Adverse events grade ≥ 3 were observed in 39.6% of the olaparib arm and 23.3% of the placebo arm, mainly anemia and fatigue, with a toxicity profile similar to that seen in other tumor types. Patient-reported global health-related quality of life was preserved, over time, with no clinically meaningful differences from baseline in either arm or between the arms.
“We conclude that a strategic approach of first-line platinum-based chemotherapy followed by maintenance olaparib treatment should become a new standard of care for patients with metastatic pancreatic cancer who have a germline BRCA mutation,” said Dr. Kindler. ■
DISCLOSURE: Dr. Kindler has served as a consultant or advisor for Aldeyra Therapeutics, Astellas Pharma, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Erytech Pharma, Five Prime Therapeutics, Ipsen, Kyowa Hakko Kirin, Merck, and Paradox Therapeutics; and has received institutional research funding from Aduro Biotech, AstraZeneca, Bayer, GlaxoSmithKline, Merck, MedImmune, Verastem, Bristol-Myers Squibb, Lilly, Polaris, Deciphera, InhibRx, and Roche/Genentech. Dr. Cole has received honoraria from Research to Practice.
REFERENCES
1. Kindler HL, Hammel P, Reni M, et al: Olaparib as maintenance treatment following first-line platinum-based chemotherapy in patients with a germline BRCA mutation and metastatic pancreatic cancer: Phase III POLO trial. 2019 ASCO Annual Meeting. Abstract LBA4. Presented June 2, 2019.
2. Golan T, Hammel P, Reni M, et al: Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. June 2, 2019 (early release online).

