The 2015 Gastrointestinal Cancers Symposium, held January 15–17 in San Francisco, attracted almost 4,000 attendees, who heard or viewed data from nearly 800 scientific abstracts and lectures. Here are our summaries of some of the many important developments from the meeting.
Bevacizumab Plus Intensive Chemotherapy
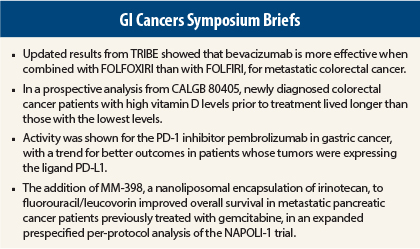
Updated results from the Italian phase III TRIBE study indicate that bevacizumab (Avastin) is more effective in metastatic colorectal cancer when combined with the more-intensive FOLFOXIRI regimen (leucovorin, fluorouracil [5-FU], irinotecan, oxaliplatin) than FOLFIRI (leucovorin, 5-FU, irinotecan).1 FOLFOXIRI plus bevacizumab extended overall survival by 4 months and doubled the 5-year survival rate vs bevacizumab plus FOLFIRI, reported Chiara Cremolini, MD, of the Santa Chiara Hospital in Pisa, Italy.
The study evaluated 508 patients randomly assigned to bevacizumab plus either FOLFOXIRI or FOLFIRI for 6 months, after which they received maintenance bevacizumab with 5-FU/leucovorin. After a median follow-up of about 4 years, median overall survival was 29.8 months in the FOLFOXIRI arm vs 25.8 months in the FOLFIRI arm (hazard ratio [HR] = 0.80, P = .030).
“Looking at the final part of the shape of the survival curves, we see that the benefit of FOLFOXIRI plus bevacizumab increases over time, and the estimated 5-year survival is 24.9% vs 12.4%, which is double that of FOLFIRI plus bevacizumab,” she said. “Based on these results, FOLFOXIRI plus bevacizumab represents a valuable option for the upfront treatment of metastatic colorectal cancer.”
FOLFOXIRI was associated with more grade 3/4 diarrhea, mucositis, neuropathy, and neutropenia but no increase in febrile neutropenia, serious adverse events, or treatment-related deaths. The intensity of FOLFOXIRI precludes its use in all patients, acknowledged Dr. Cremolini, who suggested that it is probably not suitable for those older than age 75 or younger patients with poor performance status.
High Vitamin D Levels and Survival
Newly diagnosed colorectal cancer patients with high vitamin D levels prior to treatment lived longer than those with the lowest levels in a prospective analysis of data from a phase III study,2 adding to a growing body of evidence that vitamin D has an antitumor effect, said Kimmie Ng, MD, MPD, of Dana-Farber Cancer Institute and Harvard Medical School, Boston.
“The ultimate goal is to translate this research into an effective intervention for patients,” she said.
Dr. Ng and colleagues measured the plasma levels of vitamin D (25-hydroxyvitamin D) in 1,043 patients enrolling in the Cancer and Leukemia Group B (CALGB) 80405 trial, which evaluated three different first-line treatments for advanced colorectal cancer. Their vitamin D levels ranged from a median low of 8 ng/mL to a median high of 27.5 ng/mL. The median for all patients was 17.2 ng/mL, which is below the recommended healthy range of 20 to 30 ng/mL.
Divided into quintiles according to their baseline vitamin D level, and adjusted for other prognostic factors and healthy behaviors, patients with the highest levels had a median overall survival of 32.6 months, compared with 24.5 months for those in the lowest quintile (HR = 0.67, P = .002), and a longer time to disease progression, 12.2 months vs 10.1 months (HR = 0.80, P = .02). The benefit was seen across the three treatment arms, did not differ by KRAS status, and was not ameliorated after adjustment for other prognostic factors.
Dr. Ng acknowledged that the findings do not indicate that raising a low baseline vitamin D level will improve outcomes. Randomized studies are underway to evaluate the benefit of vitamin D supplementation before and after a cancer diagnosis.
Pembrolizumab Active in Gastric Cancer
Among metastatic gastric cancer patients treated with the anti–programmed cell death (PD-1) monoclonal antibody pembrolizumab (Keytruda), a trend was observed between level of expression of the programmed death ligand PD-L1 and outcomes, KEYNOTE-012 investigators reported.3
KEYNOTE-012 evaluated the immune checkpoint inhibitor in a variety of solid tumors. The gastric cancer cohort included 39 patients whose tumors expressed PD-L1 (a requirement for eligibility), which comprised 40% of those screened. These patients mostly had received prior chemotherapy.
Patients received pembrolizumab at 10 mg/kg every 2 weeks for up to 24 months. The primary endpoint was objective response rate by independent central review. At 8.8 months’ follow-up, there was “strong evidence of anticancer activity,” Kei Muro, MD, of Aichi Cancer Center Hospital in Nagoya, Japan, reported.
Responses were observed in 22.2% by central review and in 33.3% by investigator review. These eight responses were all partial responses; stable disease was observed in another five patients (and in four patients, response was not determined). Approximately 53% of patients had some degree of tumor shrinkage.
“Six of the eight responders were still responding at the time of this analysis,” Dr. Muro noted. The median time to response was “rapid” at 8 weeks, and median response duration was 24 weeks (range, 8 to 33-plus). Median progression-free survival was 1.9 months. At 6 months, the progression-free survival rate was 24% and overall survival rate was 69%. Median overall survival has not been reached.
Patients with high PD-L1 expression (staining in the stroma or in ≥ 1% of tumor cells) showed a trend toward improved outcomes (ie, better response rates, progression-free survival, and overall survival). Dr. Muro acknowledged, however, the current trend was “weak” and requires further investigation.
The phase II KEYNOTE-059 study of pembrolizumab monotherapy or in combination with cisplatin plus 5-FU for advanced gastric cancer will be initiated early in 2015.
Nanoliposomal Irinotecan in Metastatic Pancreatic Cancer
The addition of MM-398, a nanoliposomal encapsulation of irinotecan, to 5-FU/leucovorin was shown to improve overall survival in metastatic pancreatic cancer patients previously treated with gemcitabine, in an expanded prespecified per-protocol analysis of the NAPOLI-1 trial presented by Li-Tzong Chen, MD, of the National Institute of Cancer Research, National Health Research Institutes, Taiwan.4 Investigators had previously reported data from the intent-to-treat population, showing that MM-398 plus 5-FU/leucovorin significantly improved survival.5
“Here, in the per-protocol population, the MM-398 plus 5-FU/leucovorin combination regimen achieved a median overall survival of 8.9 months, vs 5.1 months with 5-FU/leucovorin alone (HR = 0.47, P = .0018),” Dr. Chen reported.
MM-398, liposomal irinotecan injection, contains about 80,000 molecules of irinotecan stably encapsulated in a 100-nm liposome, circulates more extensively, and achieves higher levels of the active metabolite, SN-38, in the tumor, compared to conventional irinotecan. The drug has received Fast Track status from the U.S. Food and Drug Administration.
With overall survival as the primary endpoint, the open-label study randomly assigned 417 patients to one of these arms: (1) MM-398 (120 mg/m2 every 3 weeks) as a single agent; (2) 5-FU (2,000 mg/m2 over 24 hours) plus leucovorin (200 mg/m2 over 30 min) for 4 weeks followed by 2 weeks off; or (3) MM-398 (80 mg/m2 every 2 weeks) prior to 5-FU (2,400 mg/m2 over 46 hours) and leucovorin (400 mg/m2 over 30 min).
The intent-to-treat population included all patients who were randomly assigned to either MM-398–plus–5‑FU/leucovorin or 5-FU/leucovorin–alone arms (N = 236). The per-protocol population included patients who received at least 80% of the target dose in the first 6 weeks (N = 137); and the non–per-protocol population (N = 99) were those who did not qualify for per-protocol analysis.
While these results in the per-protocol population were anticipated, “what was more surprising,” according to Dr. Chen, was the overall survival benefit in the non–per-protocol population, the combination arm vs the control arm: 4.4 months vs 2.8 months (HR = 0.56, P = .0365).
The safety profile of the combination regimen was considered manageable, with the most frequent adverse events being neutropenia, fatigue, diarrhea, and vomiting. ■
Disclosure: Dr. Cremolini is a consultant for Roche, Bayer, and Amgen. Drs. Ng and Muro reported no potential conflicts of interest. Dr. Chen has received honoraria from PharmaEngine, Novartis, Merck Serono, TTY, Taiho, Ono, and Five Prime and research medications for investigator-initiated studies from PharmaEngine, Sanofi, Merck Serono, TTY, Taiho, Novartis, and Bayer.
References
1. Cremolini C, Loupakis F, Masi G, et al: FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of metastatic colorectal cancer: Updated survival results of the phase III TRIBE trial by the GONO group. 2015 Gastrointestinal Cancers Symposium. Abstract 657. Presented January 17, 2015.
2. Ng K, Venook AP, Sato K, et al: Vitamin D status and survival of metastatic colorectal cancer patients: Results from CALGB/SWOG 80405. 2015 Gastrointestinal Cancers Symposium. Abstract 507. Presented January 17, 2015.
3. Muro K, Bang Y-J, Shankaran V, et al: Relationship between PD-L1 expression and clinical outcomes in patients with advanced gastric cancer treated with the anti-PD-1 monoclonal antibody pembrolizumab in KEYNOTE-012. 2015 Gastrointestinal Cancers Symposium. Abstract 3. Presented January 15, 2015.
4. Chen L-T, Von Hoff DD, Li C-P, et al: Expanded analyses of NAPOLI-1: Phase 3 study of MM-398 with or without 5-fluorouracil and leucovorin, versus 5-fluorouracil and leucovorin, in metastatic pancreatic cancer previously treated with gemcitabine-based therapy. 2015 Gastrointestinal Cancers Symposium. Abstract 234. Presented January 15, 2015.
5. Von Hoff D, Li CP, Wang-Gillam A, et al: NAPOLI-1: Randomized phase 3 study MM-398 (nal-IRI), with or without 5-fluorouracil and leucovorin, versus 5-fluorouracil and leucovorin, in metastatic pancreatic cancer progressed on or following gemcitabine-based therapy (Abstract O-0003). Ann Oncol 25(suppl 2):105-106, 2014.