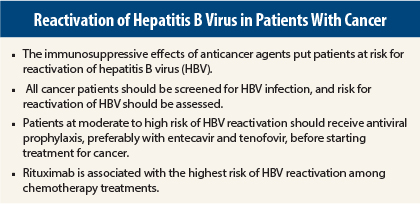
In 2015, no cancer patients should be cured of their malignancy only to die of reactivation of hepatitis B virus (HBV),” according to Anna S. Lok, MD, the Alice Lohrman Andrews Research Professor in Hepatology and Director of Clinical Hepatology at the University of Michigan, Ann Arbor.
“I recommend screening all patients before starting on chemotherapy. Assess the risk of HBV reactivation, and if the risk is moderate or high, start prophylaxis as soon as possible,” Dr. Lok told attendees at the 2015 Gastrointestinal Cancers Symposium.
“Some oncologists argue that not all patients with reactivation will have a bad outcome and ask whether we can just monitor patients and start antivirals on demand [ie, upon signs of reactivation],” she said. “But we don’t always catch things in time, and in multiple studies, outcomes for prophylaxis are better than for treatment on demand,” she explained.
Gregory J. Gores, MD, Professor of Medicine at the Mayo Clinic, Rochester, Minnesota, moderated the session and commented on Dr. Lok’s presentation. “There are probably a dozen different guidelines from multiple societies on the issue of HBV reactivation. The program committee of this meeting selected Dr. Lok to give her perspective because of her depth of knowledge in this topic and her pragmatic approach.”
Understanding HBV Reactivation
The reactivation of HBV is an abrupt increase in viral replication in a person with chronic or past HBV infection. Reactivation can occur in persons who are surface antigen–positive (HBsAg+) or surface antigen–negative (HBsAg–) and core antibody–positive (anti-HBc+) with or without surface antibody. Many patients have normal levels of liver enzymes and undetectable HBV DNA at baseline, but chemotherapy leads to immunosuppression and its sequelae: increases in HBV DNA, elevations in liver enzymes, and in some cases development of acute liver failure, and even death.
Control of HBV by the host immune response becomes weakened in the setting of immunosuppressive therapy, allowing HBV to replicate at a faster rate. It has been most extensively studied in lymphoma (with the use of the anti-CD20 antibody rituximab [Rituxan]), but it also occurs with other chemotherapy agents, corticosteroids alone (in high enough doses), biologics (antitumor necrosis factor-alpha [TNF] agents, interleukin-17, anti-CTLA4), and more classic immunosuppressive agents such as methotrexate. According to case reports, even some molecularly targeted agents, including tyrosine kinase inhibitors, the mTOR inhibitor everolimus (Afinitor), and transarterial chemoembolization with cytotoxic drugs, can cause HBV reactivation.
Special Concerns
Rituximab creates the most concern, as it carries a more than fivefold increased risk for HBV reactivation vs other regimens among HBsAg–/anti-HBc+ patients. Also concerning is the potential for delayed reactivation, up to 2 years following discontinuation of rituximab.
Reports of 109 fatalities from HBV reactivation associated with rituximab led the U.S. Food and Drug Administration (FDA) to issue a warning in 2013 and a recommendation that all patients receiving this drug be screened at baseline for HBsAg and anti-HBc, that those testing positive be monitored during and after treatment, and that antiviral prophylaxis be considered. Patients should remain on prophylaxis for 1 year after treatment ends.
Another special group includes patients with hepatocellular carcinoma, who should definitely receive an antiviral, according to Dr. Lok, “because they have underlying liver disease that led to the cancer and the data have shown that hepatocellular carcinoma patients on an antiviral have less recurrence and better survival. In this case, there are other rationales for the antiviral, not just for prevention of reactivation.”
Male gender and elevated HBV DNA at baseline indicate greater susceptibility to reactivation. An increased risk is also associated with high-intensity immunosuppressive therapy and conditioning regimens prior to bone marrow transplant, immunosuppressive therapy after solid organ transplant, high-dose steroids, and anthracyclines. HBsAg+ patients are more at risk than HBsAg–/anti-HBc+ patients.
Reactivation Seen in at Least 30% of HBsAg+ Patients
Determining the incidence of treatment-associated HBV reactivation is difficult, because clinical trials tend to exclude patients with HBV infection. “But when drugs are used in the real world, problems arise, and we have little data to guide our management,” Dr. Lok indicated.
In lymphoma patients, from whom most of the data come, Dr. Lok documented HBV-related hepatitis in almost half of HBsAg+ patients and in 4% of HBsAg–/anti-HBc+ patients. This “seemingly trivial” increase in enzymes triggered nonfatal liver failure in 4% and death in 4% of HBsAg+ patients.1
In a systematic review of patients receiving chemotherapy for various malignancies, HBV reactivation was observed in 38.7%, leading to hepatitis in 33%, liver failure in 13%, and death in 5.5% of patients who did not receive antiviral prophylaxis.2 Another study of HBsAg+ patients observed reactivation in 30%, including 58% with lymphoma and 25% with other malignancies.3
Optimizing Prophylaxis
Among the five FDA-approved nucleoside/nucleotide analogs (dosed once daily) are lamivudine (Epivir), adefovir (Hepsera), entecavir (Baraclude), telbivudine (Tyzeka), and tenofovir (Viread). Most of the data on prophylaxis are on lamivudine, the first of these agents approved.
A systematic review,2 involving 275 treated patients and 475 controls, showed that lamivudine reduced HBV reactivation and HBV-related hepatitis by 79% to 100%, reduced HBV-related liver failure by 100%, and reduced HBV-related deaths by 80% to 100%. Lamivudine-treated patients also were less likely to die of cancer, since chemotherapy interruption is less likely when reactivation is prevented, Dr. Lok noted.
The preferred agents are now entecavir and tenofovir, as they are the most potent viral suppressors and the least likely to cause drug resistance. In a recent randomized, controlled trial of rituximab-treated patients, entecavir was significantly more effective than lamivudine in HBsAg+ patients in terms of HBV reactivation (6.6% vs 30%, P = .001), HBV-related hepatitis (0% vs 13.3%, P = .003), and chemotherapy disruption (1.6% vs 18.3%, P = .002).4
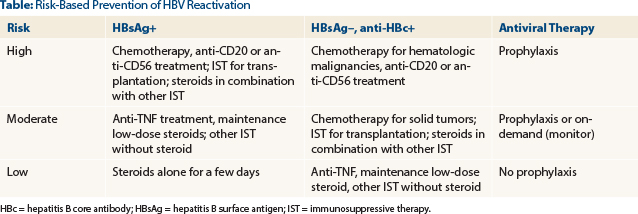
Algorithm to Guide Prophylaxis
An algorithm recently coauthored by Dr. Lok can guide clinicians in the prevention of HBV reactivation.5 It calls for all cancer patients to be screened before starting treatment and classifies patients as (1) HBsAg+; (2) HBsAg–, anti-HBc+; and (3) HBsAg–, anti-HBc–.
Risk within the first two groups is further stratified by HBV DNA levels. For HBsAg+ and HBsAg–/anti-HBc+ patients, those considered at high-risk (see table on page 16) should receive a prophylactic antiviral; moderate-risk patients can receive prophylaxis or be monitored and receive on-demand antivirals; low-risk patients can receive usual care, as can the third group.
For patients with undetectable or very low levels of HBV DNA, Dr. Lok does not advocate delaying chemotherapy. However, this step may be wise for patients with high levels, “because once patients are immunosuppressed, the response to the antiviral appears to be less robust,” she noted. ■
Disclosure: Dr. Lok has held consulting or advisory roles for Gilead Sciences and GlaxoSmithKline and has received research funding from Gilead Sciences and Bristol-Myers Squibb. Dr. Gore reported no potential conflicts of interest.
References
1. Lok AS, Liang RH, Chiu EK, et al: Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy: Report of a prospective study. Gastroenterology 100:182-188, 1991.
2. Loomba R, Rowley A, Wesley R, et al: Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med 148:519-528, 2008.
3. Yeo W, Zee B, Zhong S, et al: Comprehensive analysis of risk factors associating with hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer 90:1306-1311, 2004.
4. Huang H, Li X, Zhu J, et al: Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: A randomized clinical trial. JAMA 312:2521-2530, 2014.
5. Hwang JP, Lok AS: Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol 11:209-219, 2014.