Venous thromboembolic events are more prevalent in patients with cancer than in persons without it. Cancer is associated with a high rate of venous thromboembolism recurrence, bleeding, requirement for long-term anticoagulation, and reduced quality of life. Moreover, thrombosis is the second most common cause of death in cancer patients. It is incumbent upon clinicians, therefore, to determine which patients are at risk for venous thromboembolism and warrant prophylaxis and to know the optimal treatment for patients in whom venous thromboembolism has occurred.
The ASCO Post asked an expert in the area of anticoagulation, Jean M. Connors, MD, to share her thoughts on this important topic. Dr. Connors is Medical Director of the Hemostatic Antithrombotic Stewardship and Anticoagulation Management Services at Brigham and Women’s Hospital and Dana-Farber Cancer Institute and Assistant Professor, Harvard Medical School, Boston.
Cause for Concern
Why should we be concerned about venous thromboembolism in cancer patients?
In the average ambulatory cancer population, the overall risk of venous thromboembolism is 4% to 6%; however, this risk is clearly higher with certain tumor types—gastrointestinal cancers, especially gastric and pancreatic disease, along with lung cancer and a few others. Risk is increased for the first 3 months after diagnosis and accumulates over time. Bleeding complications are also increased in cancer patients, both with treatment and with the use of thromboprophylaxis.
We can reduce the risk of venous thromboembolism by about 50%, but there are side effects and costs associated with this strategy. That is why it is important to risk-stratify patients: to target the high-risk groups who are likely to realize the most benefit with the least harm.
Risk Stratification
So, we target patients at highest risk. How do we determine this?

Alok Khorana, MD
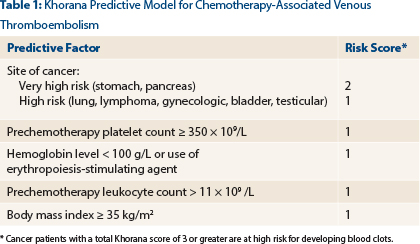
There are a number of risk-stratification scores developed to try to identify patients at significantly increased risk. The most validated and easiest to use is the Khorana score, a simple model [developed by Alok Khorana, MD, and colleagues] that predicts thrombosis risk based on a collection of baseline clinical and laboratory variables—type of cancer, BMI [body mass index], and complete blood cell count (platelet, leukocyte, hemoglobin).1
The Khorana multivariate scoring model was applied in a derivation cohort of 2,701 cancer outpatients and validated in an independent cohort of 1,365 patients and is simple to use (Table 1).1 You can compile this risk assessment score and determine the risk for the individual patient. Patients at very high risk—those with Khorana scores ≥ 3—are intuitively likely to benefit from prophylaxis.
Hospital vs Ambulatory Setting
Is the use of prophylaxis for high-risk patients restricted to the hospital setting, or is it beneficial in any group of outpatients?
Venous thromboembolism prophylaxis is indicated for hospitalized cancer patients and those undergoing cancer-associated surgery. There has been a push over the past couple of years to adapt prophylaxis for ambulatory cancer patients undergoing chemotherapy, but this remains controversial, as studies involving all types of cancer patients result in only a modest overall incidence of venous thromboembolism. The consensus is that prophylaxis should not be given to all ambulatory patients, with the exception of persons with multiple myeloma receiving treatment with immunomodulatory agents.
A study by Khorana and colleagues presented at the 2015 American Society of Hematology (ASH) Annual Meeting, however, did examine the use of the low–molecular-weight heparin dalteparin [Fragmin] in a randomized trial of 98 outpatients deemed at very high risk, with Khorana scores ≥ 3.2 Venous thromboembolic events occurred in 12% on the dalteparin arm and 21% in the control arm, showing a 9% absolute risk reduction and a 42% relative risk reduction with dalteparin (95% confidence interval [CI] = 0.23–1.89). Major bleeding was similar in each arm, but clinically relevant bleeding was higher with dalteparin. Although there was a reduction in the number of venous thromboembolic events in ambulatory patients at high risk, the decrease in venous thromboembolism was associated with increased clinically relevant (though nonmajor) bleeding (see “Selected Abstracts From 2015 ASH Annual Meeting: Part 3,” in the March 10, 2016, issue of The ASCO Post).
Patients With CNS Tumors
Is the prophylactic and therapeutic management of venous thromboembolism different in patients with primary central nervous system (CNS) tumors or metastatic tumors in the brain?
Emerging data suggest that patients with primary CNS tumors or CNS metastases do relatively well on anticoagulation, but the data are from retrospective analyses. Venous thromboembolism prophylaxis and treatment in these patients have to be highly individualized and carefully monitored. The majority of patients in these analyses have had treatment, either systemic or localized to the brain metastases, which must be considered when assessing a patient with a CNS tumor and venous thromboembolism.
A study presented by Chatree Chai-Adisaksopha at ASH 2015 evaluated low–molecular-weight heparin in patients with primary or metastatic brain tumors, compared with patients with cancers but no CNS involvement. They found no differences in recurrent venous thromboembolism, major bleeding, or clinically relevant bleeding, though intracranial bleeding occurred more frequently in patients with brain tumors.3
Catheter-Related Thrombosis
What is the recommended duration of therapy for thrombosis associated with the use of catheters?
The general consensus is to treat for 3 months even if the line is removed. But if the catheter is still present, anticoagulation is continued while the catheter remains in place.
First-Line Treatment
What is the best front-line therapy for cancer-associated venous thromboembolism?
Currently, in cancer patients with a new venous thromboembolism diagnosis, all societies endorse the use of low–molecular-weight heparin as first-line therapy during the first 3 to 6 months after the venous thromboembolism diagnosis. This is largely based on the 2003 CLOT study, which found that low–molecular-weight heparin had better efficacy than warfarin in preventing recurrent blood clots.4
During the 6-month study period, 27 of 336 patients in the dalteparin group had recurrent venous thromboembolic events, as compared with 53 of 336 patients on warfarin (hazard ratio [HR] = 0.48; P = .002). The probability of recurrent venous thromboembolism was 9% and 17%, respectively, with a risk reduction of 52%. Reanalyzing the data using competing risks methods instead of Kaplan-Meier survival rates demonstrated that the venous thromboembolism recurrence rate was still reduced by 30%. No significant differences were observed in the rates of bleeding. It is also important to note that a post hoc analysis indicated that, at 12 months (ie, 6 months post treatment, off therapy), a mortality benefit was noted for dalteparin in the subgroup of patients without metastatic disease (20% vs 36%, HR = 0.50, 95% CI = 0.27–0.95).5
Dalteparin is the only low–molecular-weight heparin that is U.S. Food and Drug Administration (FDA)-approved for monotherapy treatment of venous thromboembolism in cancer patients with once-a-day dosing. It is given as 5,000 U subcutaneously for venous thromboembolism prophylaxis and using weight-based dosing for treatment.
Fondaparinux, a synthetic pentasaccharide that is not a low–molecular-weight heparin but an indirect factor Xa inhibitor, is the most easily tolerated injection, with no associated stinging or burning; however, its long half-life of 17 hours makes it less suitable for those at risk of bleeding. Enoxaparin (a low–molecular-weight heparin) at 1mg/kg twice daily is probably the safest choice in patients at high risk for bleeding, given its short half-life. For many patients, however, we select whichever parenteral agent—enoxaparin, dalteparin, or fondaparinux—is covered by their insurance. Some cancer patients are required to pay $3,000 to $4,000 per month, which is cost prohibitive for many patients.
Available Guidelines
Do specialty and society guidelines differ in any important ways in terms of their recommendations?
We have guidelines from the NCCN [National Comprehensive Cancer Network], ASCO, the ACCP [American College of Clinical Pharmacology], ESMO [European Society for Medical Oncology], and GFTC [Groupe Francophone Thrombose et Cancer]. All of these guidelines presently state that for cancer patients with new blood clots, treat with low–molecular-weight heparin for the first 3 to 6 months. After that, the guidelines vary somewhat in terms of next-step recommendations.
Newer Anticoagulants
Do direct factor Xa or IIa inhibitors have a role in cancer-associated venous thromboembolism?
Everyone wants to use the direct oral anticoagulants in cancer patients, but we have very limited data on their safety and efficacy. For carefully selected patients, these agents should be no better or worse than low–molecular-weight heparin or warfarin. There is no doubt that they are more convenient than either warfarin or low–molecular-weight heparins.
Several studies presented at ASH 2015 evaluated the safety and efficacy of rivaroxaban (Xarelto), the first direct oral anticoagulant to become FDA-approved for the treatment of venous thromboembolism. Two separate studies showed the rate of new or recurrent venous thromboembolism was 4.3%,6,7 but major bleeding rates differed. They ranged from 1.1% in a prospective quality initiative study by Simon Mantha, MD, MPH, and colleagues (which excluded patients with active gastrointestinal or genitourinary cancer, whose risk might be higher) to 10.8% in a retrospective review from Huntsman Cancer Center (see “Selected Abstracts” in The ASCO Post, March 10, 2016). The authors of these two studies concluded that rivaroxaban may be a safe and acceptable alternative to dalteparin in the treatment and secondary prevention of cancer-associated thrombosis.
A third study presented at ASH 2015, a single-center analysis in a retrospective comparison with dalteparin by Ateefa Chaudhury, MD, et al, also showed no efficacy or safety differences between these treatments.8
One concern in the cancer population that we do not have in the general population is the potential for drug-drug interactions with direct oral anticoagulants—specifically, the effect of some chemotherapy agents on direct oral anticoagulant plasma concentrations. We need pharmacokinetic and pharmacodynamic data for these drug-drug interactions as well as efficacy and safety data to determine which cancer patients can safely receive direct oral anticoagulants to treat cancer-associated venous thromboembolism.
Long-Term Anticoagulation
Do you have any guidance for managing patients beyond 6 months?
There are really no data to guide us here. The current standard is to continue patients on anticoagulation if they have persistent cancer or remain on treatment. With the current trend toward maintenance therapy in a number of malignancies, this can mean lengthy treatment that is expensive or inconvenient for many patients.
An important study that might provide some data was presented at ASH 2015 by Chatree Chai-Adisaksopha, MD, and colleagues, demonstrating that some patients can be switched from low–molecular-weight heparin to warfarin after 6 months.9 The study evaluated data from the RIETE Registry, selecting 1,502 patients who completed 6 months of low–molecular-weight heparin, then either continued low–molecular-weight heparin or switched to warfarin at the treating physician’s discretion. They found no statistically significant difference in recurrent venous thromboembolism between these groups (HR = 0.67, 95% CI = 0.44–1.02, P = .06) and no difference in major bleeding or total bleeding, although the number of events in both groups was low (see “Selected Abstracts” in The ASCO Post, March 10, 2016).
The authors concluded that in patients with cancer-associated thrombosis who complete 6 months of anticoagulation, switching to warfarin does not increase the risk of recurrent venous thromboembolism, major bleeding, or total bleeding as compared to continuing low–molecular-weight heparin in selected patients. It showed that warfarin can be an acceptable alternative anticoagulant in patients who, for one reason or another, do not desire long-term treatment with low–molecular-weight heparin.
Role of Warfarin
Aside from the scenario of switching from low–molecular-weight heparin after 6 months, are there other situations where you would use warfarin?
I think there is also a role for warfarin in other patients: those who are on a treatment that does not affect warfarin metabolism, patients with low–thrombotic-risk tumors, those who are unable to give themselves daily injections, and patients who cannot afford low–molecular-weight heparin, which can cost $4,000 a month for those with poor insurance coverage.
For many patients with cancer, warfarin can be an acceptable anticoagulant, and we have retrospective data from the nonrandomized RIETE Registry showing that switching to warfarin at 6 months is as good as maintaining patients on low–molecular-weight heparin.9 Retrospective analysis of our data by Ariela Marshall and myself also demonstrate that for those patients deemed eligible for warfarin or for those who cannot afford or tolerate parenteral agents, the efficay and safety of warfarin are not inferior.10 Recent data from the CATCH trial also found no statistically significant difference between tinzaparin (not available in the United States) and warfarin in the upfront treatment of venous thromboembolism in cancer patients. However, the patient population in this study may have been different from that in the CLOT study.11
Real-World Practice
How are clinicians managing patients in the real world? Are they following the guidelines and practices we’ve discussed?
Despite the guidelines, in many patients it is impractical to use injectable or parenteral agents, and so, in the real world the guidelines are not always followed. In the study we discussed from the RIETE Registry, it was the clinician’s decision whether to continue low–molecular-weight heparin or switch to warfarin, and we saw that 739 of the 1,502 patients were switched.9 In these cases, the physician apparently determined the patient was a good candidate for warfarin (see “Selected Abstracts” in The ASCO Post, March 10, 2016).
At ASH 2015, we also heard, from Dr. Khorana and colleagues, an analysis of prescribing practices from a Humana health insurance claims database of 2,941 newly diagnosed cancer patients who developed venous thromboembolism and received anticoagulation in outpatient settings.12 Of them, 97% received anticoagulation—low–molecular-weight heparin (25%), low–molecular-weight heparin/warfarin (18.7%), warfarin (29%), or rivaroxaban (24.1%). The median treatment durations for low–molecular-weight heparin, low–molecular-weight heparin/warfarin, warfarin, and rivaroxaban were 3.29, 7.76, 8.12, and 7.92 months, respectively, with low–molecular-weight heparin having the lowest rate of persistence with the initial therapy (37%). The proportion of patients who switched from their initial treatment to another anticoagulation treatment was 22.9%, 8.9%, 7.3%, and 4.7% in the low–molecular-weight heparin, low–molecular-weight heparin/warfarin, warfarin, and rivaroxaban cohorts, respectively.
Warfarin and rivaroxaban were prescribed at the same rate as low–molecular-weight heparin for initial treatment of venous thromboembolism, and low–molecular-weight heparin was associated with the least persistence, shortest treatment duration, and greatest likelihood of switching. I think it’s highly instructive and should be recognized that in real-world practice, nearly 50% of patients were being treated upfront with warfarin, despite the universal recommendation for using low–molecular-weight heparin.
Cost of Care
Finally, how does the cost of anticoagulation figure into the management equation, or should it?
My colleagues Nathan Connell, MD, MPH, and Greg Abel, MD, MPH, and I aimed to characterize the effectiveness and costs associated with two management strategies for cancer-associated venous thromboembolism and presented our findings at ASH 2015.13 We concluded that while low–molecular-weight heparin is an effective treatment, it offers only a small gain in quality-adjusted life-years (QALYs) compared to warfarin, and that this gain is associated with a significant increase in cost.
In our Markov state-transition model, we found the odds ratio for recurrent venous thromboembolism while on low–molecular-weight heparin, compared to warfarin, to be 0.55 (95% CI = 0.40–0.75), but the mean cost of treatment was 10 times higher: $64,975.83 for low–molecular-weight heparin vs $6,383.39 for warfarin.
We determined that the QALY with warfarin was 1.19 and with low–molecular-weight heparin was 1.46. This resulted in a mean increase of 0.27 QALYs with low–molecular-weight heparin and an incremental cost-effectiveness ratio of $221,281.83 per QALY. At the generally accepted willingness-to-pay threshold of $100,000 per QALY, warfarin was clearly preferred from a cost-effectiveness perspective—another reason to consider warfarin a reasonable alternative to low–molecular-weight heparin in appropriate patients. ■
Disclosure: Dr. Connors received travel and accommodation expenses from Bristol-Myers Squibb for an independent review.
References
1. Khorana AA, Kuderer NM, Culakova E, et al: Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 111:4902-4907, 2008.
2. Khorana AA, Francis CW, Kuderer N, et al: Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism. 2015 ASH Annual Meeting. Abstract 427. Presented December 7, 2015.
3. Chai-Adisaksopha C, AlKindi SY, Cheah M, et al: Outcomes of low-molecular-weight heparin treatment for venous thromboembolism in patients with primary and metastatic brain tumors. 2015 ASH Annual Meeting. Abstract 428. Presented December 7, 2015.
4. Lee AYY, Levine MN, Baker RI, et al: Low-molecular-weight heparin vs coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 349:146-153, 2003.
5. Lee AY, Rickles FR, Julian JA, et al: Randomized comparison of low molecular weight heparin and coumarin derivatives on the survival of patients with cancer and venous thromboembolism. J Clin Oncol 23:2123-2139, 2005.
6. Mantha S, Miao Y, Sarasolm D, et al: Safe and effective use of rivaroxaban for the treatment of cancer-associated venous thromboembolic disease. 2015 ASH Annual Meeting. Abstract 431. Presented December 7, 2015.
7. Win KZ, Wilson N, Stenehjem DD, et al: Effectiveness and safety of rivaroxaban in treatment of venous thromboembolism in cancer patients. 2015 ASH Annual Meeting. Abstract 2319. Presented December 6, 2015.
8. Chaudhury A, Balakrishnan A, Thai C, et al: Evaluation of rivaroxaban and dalteparin in cancer associated thrombosis. 2015 ASH Annual Meeting. Abstract 432. Presented December 7, 2015.
9. Chai-Adisaksopha C, Iorio A, Crowther MA, et al: Cancer-associated thrombosis. 2015 ASH Annual Meeting. Abstract 430. Presented December 7, 2015.
10. Marshall AL, Campigotto F, Neuberg D, et al: Recurrence of venous thromboembolism in patients with cancer treated with warfarin. Clin Appl Thromb Hemost 21:632-638, 2015.
11. Lee AY, Kamphuisen PW, Meyer G, et al: Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer. JAMA 314:677-686, 2015.
12. Khorana AA, McCrae K, Milentijevic D, et al: Current practice patterns and patient persistence on anticoagulant treatments for cancer-associated thrombosis. 2015 ASH Annual Meeting. Abstract 626. Presented December 7, 2015.
13. Connell NT, Abel GA, Connors JM: Cost-effectiveness analysis of warfarin versus low-molecular weight heparin for the treatment of malignancy-associated venous thromboembolism. 2015 ASH Annual Meeting. Abstract 746. Presented December 7, 2015.


