
Steven E. Vogl, MD
Two recent publications in The New England Journal of Medicine (NEJM), and the resulting drug approval applications that have already been filed, lead to concern that the basis of medical practice on valid evidence may be corrupted. Each involves statistically shaky analysis leading to a striking result that patients with cancer and their physicians would like to be true.
Atezolizumab Plus Chemotherapy for Triple-Negative Breast Cancer
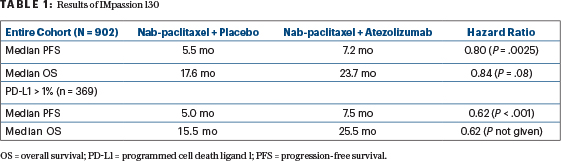
The more important of these publications, because of the enthusiastic positive reception it has generated, involves the IMpassion130 study of atezolizumab added to nab-paclitaxel for metastatic triple-negative breast cancer.1 This is a straightforward trial of single-agent chemotherapy with or without a programmed cell death ligand 1 (PD-L1) antibody. Roche/Genentech, the trial sponsor, embedded the biomarker of PD-L1 staining into the trial design and specified analysis of the entire study cohort, as well as the part of the cohort with≥ 1% PD-L1 staining, for both progression-free survival and overall survival. This plan of analysis of a full and partial cohort and two primary endpoints multiplies the number of possible comparisons yielding a positive result.
The essence of trial design is that one must define the question being asked before beginning the trial and gathering the data.— Steven E. Vogl, MD
Tweet this quote
To adjust for this difficulty, study statisticians stipulated that overall survival of the PD-L1–positive subcohort could be analyzed statistically only if the entire study cohort had a demonstrably better overall survival with the addition of atezolizumab. It turned out that the latter requirement was not met in the analysis published in 2018, but the results have been greeted with uniform acclaim as if the requirement were met (Table 1). The improvements in progression-free survival were modest but statistically significant. The improvements in overall survival were impressive but not statistically significant in the entire group, so the 10-month improvement in median survival in the PD-L1–positive subgroup could not be analyzed statistically.
It seems likely that Roche wanted to test atezolizumab in the entire population because it would double the market size (the entire metastatic triple-negative population). They expected the benefits to be most impressive among those with PD-L1–positive tumors, and took a chance that results would be significantly better in this group and that this relatively small single trial would demonstrate this.
The essence of trial design is that one must define the question being asked before beginning the trial and gathering the data. Once the data are in hand, an innovative analyst will be able to create a question with an answer that favors the interests of the trial sponsors if given the time and the ingenuity. All of us who treat metastatic breast cancer would love to improve on therapy heretofore associated with a median overall survival of about 1 year from the identification of metastases from triple-negative breast cancer. IMpassion130 so far has not established that the addition of atezolizumab causes the desired improvement. While encouraging, despite media mention of saving lives, currently available data do not provide even a hint that anyone is cured.
Roche could have designed two studies of nab-paclitaxel with or without atezolizumab and analyzed them separately, picking a single PD-L1 cutoff for entry into one or the other. This would have lengthened accrual and required more patients, but would have established whether the addition of atezolizumab improved outcomes for the PD-L1–positive population. Merck chose this path for the development of pembrolizumab as a single agent for patients with metastatic non–small lung cancer (NSCLC) and reaped the rewards of a landmark study showing the benefits of first-line pembrolizumab over chemotherapy when PD-L1 tumor proportion score was ≥ 50%.2
Defenders of atezolizumab plus chemotherapy for these women point out that a more mature analysis of IMpassion130 may show an overall survival benefit for the entire study population, allowing for the subgroup analysis of PD-L1–positive cancers to be analyzed per protocol. They also point out that studies of other anti–programmed cell death protein 1 (PD-1)/PD-L1 antibodies for metastatic triple-negative breast cancer are in progress, and these may be unequivocally positive. The skeptic points out that all these analyses, alas, may prove negative.
Unfortunate Truths
The unfortunate truth in the 21st century is that most clinical cancer research is funded by pharmaceutical companies and driven by considerations of the greatest profit resulting from the smallest expenditure. The exorbitant prices extracted from society for these new drugs, and their initial impressive activity, have resulted in unprecedented massive investment leading to rapid and broad development across disease types.
All of us who treat metastatic breast cancer would love to improve on therapy heretofore associated with a median overall survival of about 1 year from diagnosis of metastatic triple-negative breast cancer.— Steven E. Vogl, MD
Tweet this quote
If regulatory authorities grant unrestricted approval for atezolizumab added to nab-paclitaxel for women with metastatic triple-negative breast cancer, every drug company will be tempted to design less expensive, smaller studies with complicated statistical designs with the intention of violating the designs if this appears to be the quickest and cheapest route to approval – why not? A potentially harmful precedent will be set. Designs like that of IMPassion 130allow smaller accrual, enable faster analysis, and spend less money. Even if the addition of atezolizumab turns out to be a true “winner” in metastatic triple-negative breast cancer, eventually this corruption of the process will back an expensive and toxic loser.
Can we rely on “the market” to judge whether atezolizumab should be added to chemotherapy for metastatic triple-negative breast cancer? Probably not—drugs approved by the U.S. Food and Drug Administration (FDA) are often considered the standard of care. In fact, many experts in the breast cancer community have already stated they think atezolizumab plus chemotherapy is the standard of care for PD-L1–positive metastatic triple-negative breast cancer, calling the combination “a major milestone.” Many of these experts participated in IMpassion130. Entities run for income or profit will judge atezolizumab not only by the quality of the data and its analysis, but by the net income the drug’s employment can generate. If it loses money, such organizations will refuse to let their physicians administer it. We can rely on the trial sponsor to ensure that the latter circumstance will not prevail for major users.
It would be folly for the FDA to approve atezolizumab plus chemotherapy for PD-L1–positive metastatic triple-negative breast cancer without restriction, as a properly done study demonstrating major benefit is still lacking. More acceptable alternatives are to wait for a trial in the target population to be positive for overall survival or to grant immediate temporary approval, with permanent approval conditional on positive results of another study of added atezolizumab targeting overall survival in a PD-L1–positive population.*
Nivolumab Plus Ipilimumab in Creative Analysis
Bristol-Myers Squibb (BMS) did exactly what I propose for Roche in studying its drugs nivolumab and ipilimumab for the treatment of patients with metastatic non–small cell lung cancer (NSCLC). In CheckMate 227 it conducted separate three-arm studies according to PD-L1 expression (under or over 1%).3 The design of each of the two studies makes clear the goal of demonstrating that nivolumab plus ipilimumab is superior to each of two other arms. Both are very expensive drugs sold by BMS. The NEJM paper2 reported that “external evidence” led to a protocol amendment, which stipulated analysis of the results of two arms of each study (one for those PD-L1–positive and one for those PD-L1–negative) be combined according to a new parameter—tumor mutational burden. This decision was made after accrual to both studies was complete, but before a data lock for analysis.
A cynic would suspect that the results of the studies as originally written were not favorable to BMS, though I know of no direct evidence for this. To my knowledge, the results of each three-arm study have not been completely presented or published. The only other information I could find on the results of CheckMate 227 shows improved progression-free survival from nivolumab with chemotherapy compared to nivolumab alone in a fragment of the study for subjects with PD-L1 < 1%. Dr. Hossein Borghaei presented this analysis at the 2018 ASCO Annual Meeting.4
Choice of a method of tumor mutational burden analysis and the cutoff for positive or negative values were made after all patients had entered the trials. According to the NEJM paper, BMS chose a cutoff of 10 mutations per megabase for the analysis of nivolumab plus ipilimumab compared to chemotherapy, but 13 mutations per megabase for a promised analysis of progression-free survival comparing nivolumab alone compared to chemotherapy. A cynic would suspect that decisions to rewrite the study and choose a new sorting biomarker and its cut points might be self-serving.
Of 1,739 patients in the 2 studies, 1,004 had a tumor mutational burden that could be determined retrospectively, 444 had at least 10 mutations per megabase, and 299 of the latter group were randomly assigned to either the nivolumab-plus-ipilimumab arm or the chemotherapy-alone arm and included in the NEJM paper. One-year progression-free survival was 13% for chemotherapy only and 43% for nivolumab plus ipilimumab (nominal P value < .001).
The FDA had deferred a decision on whether to approve nivolumab plus ipilimumab for lung cancer with a high tumor mutational burden until May 2019, requesting more nearly complete overall survival information. In January 2019, BMS withdrew the application pending a final survival analysis of portions of CheckMate 227 that would satisfy the FDA’s request for more survival data. The news release announcing the withdrawal makes no mention of statistical regrets.
The [nivolumab-plus-ipilimumab] analysis is interesting and generates hypotheses that are worth testing in carefully designed clinical trials carried out to completion according to their original designs.— Steven E. Vogl, MD
Tweet this quote
I believe BMS should get one award for creative rewriting of a trial that was essentially complete, and a second award for effectively marketing a combined analysis of parts of 2 studies sorted by a new biomarker to find a small population (17% of the total entered in the 2 studies) that seems to benefit from 2 of its very expensive drugs.
The published analysis is interesting and generates hypotheses that are worth testing in carefully designed clinical trials carried out to completion according to their original designs. Validation of the tumor mutational burden testing methods and cutpoints should be required before the trials begin.
The creativity of BMS in repurposing a study for which it had already paid to prove the utility of two of its expensive drugs given together to a newly defined subgroup of patients with lung cancer is impressive. It should not be rewarded by approval of a new indication. That creativity should first lead to a carefully designed and conducted study to confirm the benefits and to justify the toxicity of combined nivolumab plus ipilimumab, as well as to confirm the validity of the chosen biomarker technique to define the study population. Awarding an FDA indication based only on the currently available subgroup analyses would encourage similar pharmaceutical drug development practices in the future that will tend to produce false-positive results that do not benefit our patients. ■
*Editor’s Note: On March 8, 2019, as we were preparing this issue to go to press, the FDA granted accelerated approval to atezolizumab plus nab-paclitaxel for the treatment of adults with unresectable, locally advanced or metastatic, PD-L1–positive triple-negative breast cancer, based on data from the -IMpassion130 trial. The accelerated approval of the atezolizumab combination in this setting is contingent on the results of a confirmatory trial.
At Microphone 1 is an occasional column written by Steven E. Vogl, MD, of the Bronx, New York. When he is not in his clinic, Dr. Vogl can generally be found at major oncology meetings and often at the microphone, where he stands ready with critical questions for presenters of new data.
The opinions expressed in this column are those of the author. If you would like to share your opinion on this or another topic, please write to editor@ASCOPost.com.
DISCLOSURE: Dr. Vogl owns stock in Bristol-Myers Squibb.
REFERENCES
1. Schmid P, Adams S, Rugo HS, et al: Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108-2121, 2018.
2. Reck M, Rodriguez-Abreu D, Robinson AG, et al: Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833, 2016.
3. Hellman MD, Ciuleanu TE, Pluzanski A, et al: Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 378:2093-2104, 2018.
4. Borghaei H, Hellman MD, Paz-Ares LG, et al: Nivolumab + platinum-doublet chemotherapy vs chemo as first-line treatment for advanced non-small cell lung cancer with < 1% tumor PD-L1 expression: Results from CheckMate 227. 2018 ASCO Annual Meeting. Abstract 9001. Presented June 4, 2018.

