The anti–PD-1 inhibitor MK-3475 (formerly lambrolizumab) is in late-stage trials for advanced melanoma and is also being studied in other malignancies, including non–small cell lung cancer (NSCLC). An important aspect of Merck’s development program for MK-3475, as well as for other anti–PD-1 agents being developed by other pharmaceutical companies, is identification of a biomarker for patient selection.
KEYNOTE-001 Trial
As reported at the Annual Meeting of the American Association for Cancer Research (AACR), separate analyses of cohorts treated with MK-3475 in the KEYNOTE-001 phase I trial sought to correlate response to this agent with the biomarker PD-L1. A potent antibody that inhibits PD-1, MK-3475 is designed to restore the ability of the immune system to recognize and target cancer cells by selectively achieving dual blockade of two ligands of the PD-1 protein: PD-L1 and PD-L2. PD-L1 is expressed in some tumors both by tumor cells and by infiltrating immune cells within the stroma.
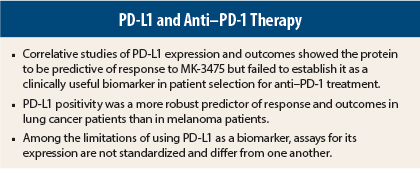
Taken together, results of these analyses suggest that PD-L1 may be an important biomarker in patients receiving treatment with MK-3475, but further studies are needed for the protein to be established as clinically useful for patient selection. That said, the results were more promising for clinical utility of PD-L1 in NSCLC than in advanced melanoma, based on the differential response between patients with strong PD-L1 expression vs weak or negative expression.
A major problem is the lack of standardization of PD-L1 testing. Different pharmaceutical companies developing anti–PD-1 immunotherapies use different assays, making it difficult to compare results, noted Mario Sznol, MD, of Yale Cancer Center, New Haven, Connecticut, who discussed both correlative trials at a Clinical Trials Symposium (see sidebar).
Melanoma Substudy
KEYNOTE-001 had a complex design with many cohorts. The melanoma cohorts included 195 patients with advanced disease. After a mandatory biopsy, patients were treated with one of three doses of MK-3475 for 12 weeks; response was assessed and responders continued on treatment until disease progression, whereas nonresponders were dropped from the study. Lead author of the melanoma correlative study was Adil Daud, MD, of the University of California, San Francisco, Helen Diller Family Comprehensive Cancer Center.
Previous treatment with ipilimumab (Yervoy) was allowed. Those who had received ipilimumab had no restriction on prior therapies; those who were ipilimumab-naive could have had up to two prior therapies.
Of 125 evaluable patients, 89 (71%) had PD-L1–positive tumors, and 36 (29%) were PD-L1–negative. The overall response rate was 40%. Among PD-L1–positive patients, the response rate was 49% vs 13% among PD-L1–negative patients (P = .0007).3
Durable responses were observed in both PD-L1–positive and PD-L1–negative patients, Dr. Daud said. This limits the clinical utility of using PD-L1 expression as a biomarker in advanced melanoma, he noted.
Progression-free survival curves revealed major differences between PL-L1–positive and –negative patients. Median progression-free survival was 10.6 months in PD-L1–positive patients vs 2.9 months in PD-L1–negative patients (P = .0051). Overall survival data are not yet mature.
Data from ongoing studies of MK-3475 should provide additional information on the correlation between PD-L1 expression and response in patients treated with MK-3475. Ongoing trials in advanced melanoma include the phase II KEYNOTE-002 trial and the phase III KEYNOTE-006 trial.
NSCLC Cohort
A separate analysis of patients with NSCLC treated with MK-3475 showed that PD-L1 expression in at least 50% of tumor cells predicted response to MK-3475. These findings were based on analysis of a training set of 146 NSCLC patients from the ongoing KEYNOTE-001 phase I study and were reported by Leena Gandhi, MD, PhD, of Dana-Farber Cancer Center, Boston.
Using the 50% cutoff point, about 25% of patients were strongly positive for PD-L1 expression. The overall response rate for both PD-L1–positive and –negative groups was 19%. However, strong positivity clearly differentiated responders from nonresponders, Dr. Gandhi said.
Median progression-free survival was 14.1 weeks in the PD-L1–positive patients vs 9.3 weeks in the weakly positive (1%–49% cutoff) and negative patients. Overall survival was 9.3 vs 7.3 months, respectively, which was not statistically significant.
“Progression-free survival was markedly improved in those with strong PD-L1 staining, and responses were durable. We expect that the median and final progression-free survival may change over time because the data to date include only very few patients with long-term follow-up. As with melanoma, there is no statistically significant difference in survival at this point, but we do see a trend favoring PD-L1 positivity,” Dr. Gandhi said.
Additional analysis will be done on expanded patient groups in the phase I study, and an ongoing randomized study will compare MK-3475 at 2 mg/kg vs docetaxel at 10 mg in NSCLC. MK-3475 is also being evaluated in multiple trials across more than 30 types of cancer and hematologic malignancies. ■
Disclosure: Dr. Gandhi reported no potential conflicts of interest.
References
1. Daud AI, Hamid O, Ribas A, et al: Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma: Correlation of tumor PD-L1 expression with outcome. AACR Annual Meeting. Abstract CT104. Presented April 6, 2014.
2. Gandhi L, Balmanoukian A, Hui R, et al: MK-3475 (anti-PD-1 monoclonal antibody) for non-small cell lung cancer (NSCLC): Antitumor activity and association with tumor PD-L1 expression. AACR Annual Meeting. Abstract CT105. Presented April 6, 2014.