Clinical research is vital for the development and improvement of methods designed to prevent and treat cancer. The majority of clinical trials take place in the developed world through sponsored pharmaceutical research companies.1 The corresponding lack of research in developing countries results in two unmet needs related to cancer treatment: First, recommended treatments do not reflect ethnic (genetic), cultural, environmental, and resource differences between developed and developing countries. Second, there is little research conducted on common tumors found primarily in developing countries. Consequently, the ability to diagnose and treat these diseases is impaired in many parts of the world.
Research Barriers
The most frequent barriers to successful clinical research in South America are the lack of national or regional organizations linked to cancer research, lack of public recognition, and lack of funding capabilities, coupled with a heavy dependence on pharmaceutical companies that perform clinical research and scarcity of funding outside these companies.2
Nevertheless, it is critical to promote clinical research in developing countries, with training of research professionals at all levels (data managers, research nurses, etc) according to the local context, and identification of innovative resources to fund research. It is also critical to develop new models to answer questions that are relevant to our own patient populations and clinicians, being at the same time useful and cost-effective for each health-care system.3
Cancer research must be framed in a locoregional context because, frequently, the extrapolation of methodologies from developed countries may not be fully applicable, and the interpretation of results from the international literature may sometimes be erroneous when applied to different ethnic and regional populations.4
It is also necessary to promote not only clinical research, but also basic, translational, epidemiologic, and implementation research. This is essential not only for patient care, but also for the development of national cancer plans and the creation of better health-care systems.
Publicly Funded Clinical Trials
The most important objective of a publicly funded clinical cancer trial is the identification of optimal therapies. This differs from the goals of pharmaceutical and biotechnology companies—rapid evaluation of their innovative products and market authorization if they demonstrate effectiveness, with a secondary aim of finding the best integration of active new agents into cancer treatment regimens for a variety of cancer sites, age groups, and clinical scenarios.5
These differences arise because drug approval and profitable return on investment are the fundamental objectives of pharmaceutical companies, whereas independent research may have broader objectives, including the answer to clinical questions or the validation of scientific hypothesis. Many important cancer trials do not contain experimental agents and therefore lack a pharmaceutical sponsor. This is also true for studies that intend to answer a purely clinical question, epidemiologic studies, and, more recently, implementation research.
Another critical objective for publicly funded trials is to identify the most effective treatments that national health-care systems should make available (and, likewise, to identify ineffective treatments that health-care systems should not support). In this context, health-care coverage should be adapted to different resource levels.
The development of evidence-based guidelines and recommendations for clinical practice are highly dependent on local conditions, so it is also imperative to promote independent research for this purpose at the national level.
Publicly funded trials can determine whether interventions found to be effective in other countries are also effective in one’s own country. We know that health-care systems vary from country to country and that national populations vary by age structure, comorbidity, and genetic background. Particularly with the newer generation of biologic agents, we need to establish whether treatment effect or toxicity varies according to population genetics.
Further Benefits of Publicly Funded Trials
Institutional participation in clinical trials improves quality of care for all patients treated at that site. Doctors and nurses who participate in clinical trials are more likely to adopt effective innovations into routine practice, as well as to practice cancer care more rigorously.
Another potential benefit from publicly funded trials is the incorporation of translational research components into cancer trials. These additional elements may include epidemiology, development and validation of prognostic markers, evaluation of biologic mechanisms, novel imaging, cost-effectiveness, and efficacy of interventions in selected populations. All these extra components have the potential to add great value to an individual trial. In many cases, pharmaceutical companies may not be willing or able to support these added objectives.
Publicly funded trials facilitate the translation of research discoveries from academia into clinical practice. Too often, investigators at publicly funded research institutes and universities are not able to bring new discoveries into clinical evaluation because they do not have easy access to clinical trials and may not be able to stimulate interest from pharmaceutical and biotechnology companies in performing them.
In addition, publicly funded trials can expand the opportunities for evaluation of novel interventions from industry. Often, companies may only be able to underwrite evaluation of their novel agents in the most common cancers. By collaborating with a publicly funded network, companies can facilitate the evaluation of their new agent in less common cancers, as well as in age groups that are more difficult to study, such as children and the elderly.
Typically, companies see other firms as rivals and do not consider combination strategies. Publicly funded cancer networks can encourage the evaluation of a combination of novel agents from several different companies and foster collaboration.
The presence of an international publicly supported clinical trials group attracts interest from international pharmaceutical companies both for potential collaboration and to increase market share for their new agents. Companies are attracted by the existence of a clinical trials network with proven capability and expertise. Regional and national data collected by the network can also be useful for developed countries, for example, by being applicable to minorities living in many parts of the world.
Conducting Clinical Trials Globally
The world distribution of cancer patients shows that 61% live outside the United States, Europe, and Japan. Latin America has 10% of the world cancer patient population, 30% are in Asia, and 28% in China.6 Thus, the issue of trial placement has become increasingly complex. Research costs are rising, and accrual is strongly related to the size of the population. In this regard, the international patient population represents an extraordinary source of information.
It is also important to consider that regulatory processes are evolving and that harmonized regulatory requirements should be promoted. Global trials include demographics and epidemiology together with ethnicity issues. Today, it is possible to perform trials faster and better on a global scale. At the local level, knowledge and technology transfer are improved, and local expertise, together with global and local publications, are stimulated.
Additional reasons to conduct clinical trials globally are improvements in several aspects related to medical research: good clinical practices, dossier review, regulatory authorization, quality control systems, and established processes. Independent research and publicly funded trials can promote innovative regulatory processes, facilitating the development of studies that are not just focused on drug approval.
Global efforts promoting independent clinical research should include an increase in participation in phase I and II studies, explore opportunities for early-phase drugs, strengthen collaboration with academic cancer research centers, increase partnership with other cancer research organizations, and further continuous education and training programs. In addition, investment must be expanded in exploratory and proof-of-concept trials, especially those that are relevant for the different world populations and that reflect various resource levels.7
Changing the Paradigm
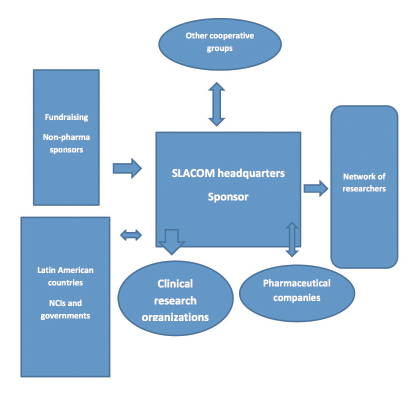
The Latin American and Caribbean Society of Medical Oncology (SLACOM) is a regional organization with more than 2,000 members (Fig. 1). Founded in 2002, SLACOM is headquartered in Buenos Aires, and it has a Board of Directors constituted by representatives from six countries of the region. Like the European Society for Medical Oncology (ESMO) or the American Society of Clinical Oncology (ASCO), SLACOM is a scientific society with individual membership, and its main activities are related to producing scientific knowledge and providing medical education.
The existing difficulties and barriers for independent research were the principal factors in SLACOM’s decision to develop a model adapted to regional conditions and feasible to implement with existing resources and capabilities. This decision resulted in the launch of a Clinical Research Institute in 2008.
The usual model for clinical research has pharmaceutical companies acting as sponsors and, in order to facilitate the process, clinical research organizations contracted for all operational matters, logistics, and administrative support. SLACOM integrates all
clinical trial players—sponsor, researchers, clinical research organizations, and patients—in a coordinated and efficient way, avoiding duplications, facilitating processes, lowering costs, and bridging gaps (Fig. 1).
Patients and researchers are central components of the strategy in SLACOM’s research activities. Other important factors in the equation are the quest to answer clinical questions, provide good epidemiologic information, understand ethnic and regional variations, and address issues related to affordability, sustainability, and applicability. The evaluation of new therapeutic strategies is frequently a key area of interest for independent groups involved in the process.8
SLACOM’s approach allows that the sponsor (or a consortium of sponsors), working alone or in partnership with other interested partners (governments, academies, health-care systems, pharmaceutical companies, etc), may be included in a hybrid model. Additional sponsors can be integrated, if they agree to follow the criteria and methodology of SLACOM researchers.
Under this structure, the clinical research organization’s work is coordinated with the group of researchers. In this context, it is important to understand that the relationship with the researcher is managed not only through the clinical research organization representative (a frequent situation in developing countries). In addition, SLACOM’s Clinical Research Institute team has continuous contact with clinical research organization members and researchers in a coordinated, personal, and functional way.
Feasibility Assessments
Prestudy feasibility analysis is a vital element in our strategy. We only select researchers who are expert in a given disease with high possibilities of patient accrual. We develop our own databases and conduct data analysis with information from more than 12 countries in Latin America. The data include each center’s capabilities, potential patient availability, and real possibilities of accrual to avoid or diminish incorrect estimations.
It is well known that the cost of a trial increases exponentially with the number of sites. Therefore, we perform a very careful analysis of the number of sites with respect to the total necessary number of patients and countries.
A timeline assessment is also included in our feasibility estimations. The use of “megasites [specialized cancer centers with a high number of patients and more possibilities for fast accrual],” selection of countries according to a very strict evaluation process, and involvement of researchers who are committed to the study are the backbone of our strategy. We also perform a thoughtful analysis of competing studies—two or more investigations competing for the same target population—to avoid these situations.
All activities are simplified in a practical and operational manner, lowering costs and improving efficiency, accrual, and quality. All the processes that are part of the research are reformulated and monitored. All contracts, drug supplies, logistics, images, hospital costs, research fees, and other expenditures are carefully managed and take into consideration the best option in terms of price-performance ratio.
Currently, we are launching the first multinational randomized phase III study in Latin America, designed to answer a clinical question and funded only though private resources. In this case, SLACOM is the only sponsor. The next steps on our agenda are the development of our own data management team, database, and tumor bank.
In Conclusion
Clinical research must be promoted globally for the benefit of the international community of cancer patients. The development of experiments and efforts trying to advance innovative models and different strategies for research must be encouraged. We need less expensive and faster models but, most importantly, studies that will answer clinical questions relevant to the different needs of world populations. Drug development and science are crucial; however, it is also critical to have additional information that will improve cancer care and make health-care systems more efficient.
The responsibility for better cancer research should be based not only on the efforts of pharmaceutical companies. Researchers, governments, nongovernmental organizations, the private sector, patient organizations, and society as a whole must be part of this process.
We strongly believe that if our research model is successful, it will improve the performance and competitiveness of South American research groups, provide information from, and for, our own patient population, and allow us to answer questions that will otherwise remain difficult or impossible to resolve.
In addition, we hope that our Clinical Research Institute, run and managed by a regional oncology society, will serve as an inspiration for similar models to be considered, expanded upon, and adapted in other regions of the world. ■
Disclosure: Funding for the phase III study is provided by a research grant from The Breast Cancer Research Foundation (BCRF).
References
1. U.S. National Cancer Institute: Find international clinical trials. Available at www.cancer.gov/clinicaltrials/international/collaborate/findtrials/findtrials. Accessed April 24, 2014.
2. Sergua B, Sadikov A, Cazap EL, et al: Barriers and challenges to global clinical cancer research. Oncologist 19:61-67, 2014.
3. Kerner JF, Cazap E, Yach D, et al: Comprehensive cancer control-research & development: Knowing what we do and doing what we know. Tumori 95:610-622, 2009.
4. Piccart M, Goldhirsch A, Wood W, et al: Keeping faith with trial volunteers. Nature 446(7132):137-138, 2007.
5. Trimble EL, Abrams JS, Meyer RM, et al: Improving cancer outcomes through international collaboration in academic cancer treatment trials. J Clin Oncol 27:5109-5114, 2009.
6. U.S. National Institutes of Health: ClinicalTrials.gov. Clinical trials, trends, charts, and maps. Available at www.clinicaltrials.gov. Accessed April 24, 2014.
7. Siegfried N, Steinhausen K, Ren J, et al: Global core competencies for clinical trials. Lancet 380:728, 2012.
8. Organisation for Economic Co-operation and Development: OECD recommendation on the governance of clinical trials. Available at www.oecd.org/sti/sci-tech/oecdrecommendationonthegovernanceofclinicaltrials.htm. Accessed April 24, 2014.
Dr. Cazap is founder and first President of the Latin American and Caribbean Society of Medical Oncology (SLACOM), Buenos Aires; former President of the Union for International Cancer Control (UICC), Geneva; a member of the International Clinical Trials Working Group (ICTW-ASCO), Alexandria, Virginia; and a member of the Board of Directors of the National Cancer Institute of Argentina (INC).
The author would like to thank Simon Gozar (SLACOM Chief Executive Officer), Daniel Campos (SLACOM Director of Research), Martine Piccart-Gebhardt (ESMO President), Alexandru Eniu (ESMO Emerging Countries Committee Chair), and Christian Dittrich (ESMO Faculty Group Coordinator for Principles of Clinical Trials and Systemic Therapy) for their helpful comments on earlier drafts of this manuscript. A special recognition goes to Margaret (Peg) Mastrianni, Deputy Director and Chief Program Officer of The Breast Cancer Research Foundation, Larry Norton, MD, and Cliff Hudis, MD, from Memorial Sloan Kettering Cancer Center for their support of SLACOM research activities.