GUEST EDITORS

Syed A. Abutalib, MD

Uroosa Ibrahim, MD
Dr. Abutalib is Associate Director, Hematology and Cellular Therapy Program and Director, Clinical Apheresis Program Cancer Treatment at Centers of America, Zion, Illinois; Associate Professor, Rosalind Franklin University of Medicine and Science; and Founder and Co-Editor of Advances in Cell and Gene Therapy. Dr. Ibrahim is a Clinical Fellow in the Stem Cell Transplantation and Cellular Therapy Program at The Mount Sinai Hospital, New York.
The ASCO Post is pleased to present Hematology Expert Review, an ongoing feature that quizzes readers on issues in hematology. In this installment, Drs. Abutalib and Ibrahim explore the current state of the art in gene therapy.
Somatic gene therapy is a novel form of drug delivery that enlists the synthetic machinery of the patient’s non-germline cells to produce a therapeutic agent. This approach has provided treatment options for diseases that are beyond the reach of traditional medicine. Applications of gene therapy are not limited to rare, inherited disorders but can potentially extend to common acquired disorders, including acquired immunodeficiency syndrome and cancer. With more than 800 cell- and gene-therapy strategies in clinical development, it seems likely that more therapies will follow, with much broader implications for the future practice of medicine.1,2
Question 1
The goal of gene therapy for genetic diseases is to achieve durable expression of the therapeutic gene (“transgene”) at a level sufficient to ameliorate or cure disease symptoms or phenotypes with negligible adverse event(s).
Which statement about gene therapy is correct?
A. The gene of interest may be transported by a vector to the target cell that must express the therapeutic protein.
B. In vivo gene therapy is based on the transfer of the vector carrying the therapeutic gene into precultured cells from the patient.
C. The intravenous route is the ideal method of vector-gene construct delivery.
D. The cost of gene therapy is not a barrier to its commercialization.
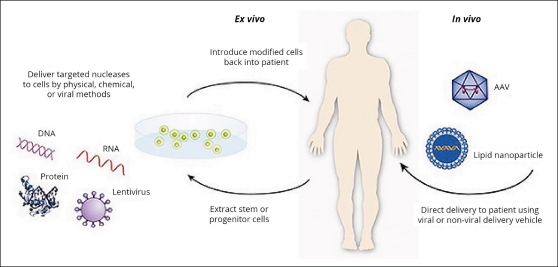
FIGURE 1: In the ex vivo approach, cells are genetically engineered and characterized in tissue culture before being implanted in the body. In the in vivo approach, the gene is delivered and expressed in situ within the body. AAV = Adeno-Associated Vector. Reproduced from the U.S. Food and Drug Administration: What is gene therapy? 2018.
Question 2
Gene therapy is no longer just a theory; several gene therapy products have been approved by the U.S. Food and Drug Administration (FDA) and the European Medical Association (EMA).
All of the following conditions have a gene therapy product approved by the FDA except?
A. Diffuse large B-cell lymphoma
B. Melanoma
C. Beta-thalassemia
D. Acute lymphoblastic leukemia
Question 3
It is well recognized that there is a lack of a single best genetically modified gene-transfer vector.
All of the following are desired qualities in a gene-transfer vector except?
A. It should display efficiency in the release of the target gene.
B. It should correct the disease phenotype.
C. It should be recognized by the immune system.
D. It should provide appropriate regulatory elements.
Question 4
Improvements in the gene-transfer vectors (“gene-delivery system”) used in therapeutic trials have led to substantial clinical successes in patients with serious medical conditions.
Which of the following statements about gene-transfer vectors is correct?
A. Retrovirus vectors infect nondividing human cells.
B. Lentivirus vectors infect dividing and non-dividing human cells.
C. Adenovirus vectors always remain extrachromosomal (episomal vector).
D. Non-viral vectors are preferred over viral vectors in human gene therapy.
Question 5
Gene therapy has come a long way over the past 50 years. Several serious obstacles have been recognized and are being addressed to allow efficient and safer gene therapy.
All of the following are potential obstacles of gene-vector transfer systems except?
A. Insertional mutagenesis
B. Immune recognition
C. Gene silencing
D. Permanent expression
Question 6
The advancement in genome-editing technology with programmable artificial nucleases, including zinc-finger nuclease (ZFN), transcription activator-like effector nuclease (TALEN), and clustered regularly interspersed short palindromic repeats (CRISPR)-associated RNA-guided endonuclease Cas9 (CRISPR/Cas9), has spurred additional optimism for potentially curing difficult-to-treat disorders.
Which of the following statements about gene-editing systems is correct?
A. Immunogenicity to the corrected gene product is not a concern with these systems.
B. These technologies do not have the ability to delete abnormally expressed genomic
aberrations.
C. Off-target toxicity is a serious concern with these systems.
D. Insertional mutagenesis remains a major concern with these systems.
Answers to the quiz appear below.
Answers to Hematology Expert Review Questions
Question 1
Which statement about gene therapy is correct?
Correct answer: A. The gene of interest may be transported by a vector to the target cell that must express the therapeutic protein.
Expert Perspective
In the vector-gene construct system, the gene of interest must be transported by a vector to the target cell that must express the therapeutic protein. Broadly, vector-gene therapy can be distinguished by two types of approaches. The in vivo approach is based on the introduction of a therapeutic gene into a vector, which is then administered directly to the target tissue in the patient (eg, injection beneath the neural retina). The vector will transfer the gene of interest into the target tissue (“vector transduction”) to produce the therapeutic protein (eg, retinal pigment epithelium–specific 65 kDa protein). The ex vivo approach is based on the transfer of the vector carrying the therapeutic gene into cultured cells, usually from the patient. Subsequently, the genetically re-engineered cells are re-introduced to the patient, where they express the therapeutic protein (eg, autologous CD19 chimeric antigen receptor [CAR]-transduced T cells1-9; Figure 1).
Depending on the disease to be treated (“target cell/tissue”), the therapeutic gene must be directed at target cells in specific tissues or organs (eg, brain, liver, skin). For example, in the case of blood cancers, CAR T cells are injected directly into the bloodstream.3 In the case of biallelic RPE65 mutation–associated retinal dystrophy, however, the gene of interest is injected beneath the neural retina.1-4 Thus, the cell producing the functional protein acts as a factory to safely deliver the therapeutic protein, resulting in reversal of the disease phenotype. The cost of gene therapy has been flagged as an issue that may hinder its progress as a commercially viable therapeutic.1
Question 2
All of the following conditions have a gene therapy product approved by the FDA except?
Correct answer: C. Beta-thalassemia.
Expert Perspective
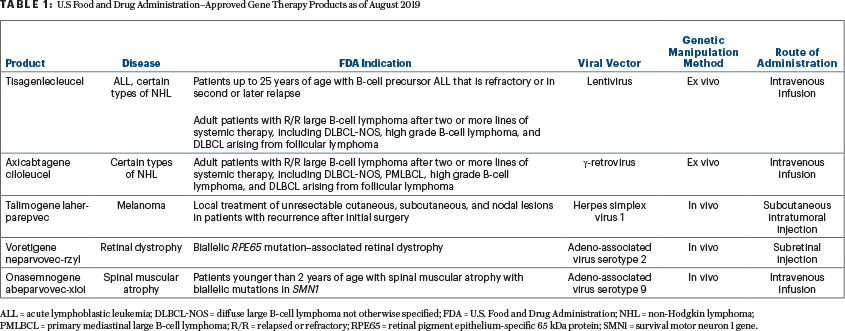
Currently, the FDA, as opposed to the EMA, has not approved gene therapy for beta-thalassemia (lentivirus vector), lipoprotein lipase deficiency (adeno-associated 1 vector), adenosine deaminase–deficient severe combined immunodeficiency (retrovirus vector), and post-haploidentical transplant leukemia/lymphoma relapse (retroviral vector). Choices A, B, and D are correct (Table 1). The FDA has approved gene therapy for selected patients with diffuse large B-cell lymphoma, transformed large B-cell lymphoma, primary mediastinal large B-cell lymphoma, acute lymphoblastic leukemia, recurrent melanoma, biallelic RPE65 mutation–associated retinal dystrophy, and spinal muscular atrophy.10
Question 3
All of the following are desired qualities of a gene-transfer vector except?
Correct answer: C. It should be recognized by the immune system.
Expert Perspective
A gene-transfer vector that is used to release the gene must be tissue-specific, efficient in the release of one or more gene(s) of the size(s) necessary for clinical application, and must not be recognized by the host immune system. Also, it should have the ability to be purified in large quantities and high concentrations to be produced and made available on a large scale.1,4-9
Once the genetically modified gene-vector enters into the target cell or tissue, it must not induce an allergic reaction or an inflammatory process; it should increase the normal functions, correct deficiencies, or inhibit deleterious activities. Furthermore, it should be safe not only for the patient, but also for the environment and the professionals who manipulate it or are exposed to it.4-9
Finally, the vector should be capable of expressing the gene of interest (transduction efficiency), in general, for the patient’s entire life, especially in cases of inherited disorders (permanent expression). An important aspect of a gene-delivery system might be its ability to regulate the expression of the introduced gene. In diseases such as hemophilia B, strict regulation of the new gene is not as critical, given the wide range of therapeutic window for factor IX levels, but such diseases are rare exceptions. In most other diseases, strict gene regulation is desirable; indeed, constitutive expression of the introduced gene may be detrimental, or even life-threatening, and underexpression of the gene product is also considered a failure. To overcome these challenges, yeast-gene or bacterial gene regulatory systems have been adapted in vector-gene therapy.1,4-9
Question 4
Which of the following statements about gene-transfer vectors is correct?
Correct answer: B. Lentivirus vectors infect dividing and nondividing human cells.
Expert Perspective
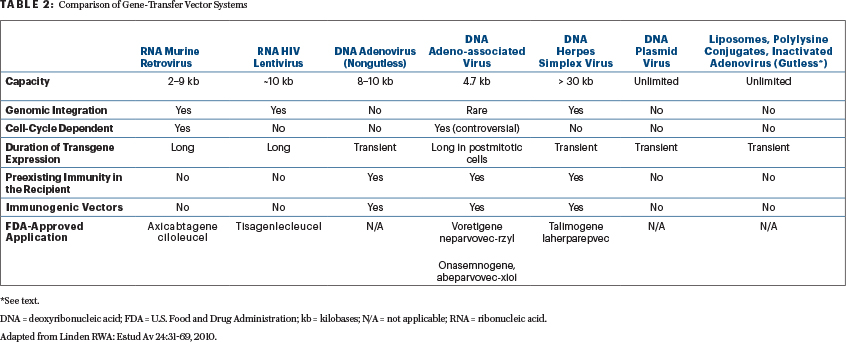
Viruses are the most common delivery method in gene therapy due to their ability to access cells and insert their genetic material (“nature’s genetic engineers”).1-9 Of the viral vectors mentioned, only retrovirus vectors are unable to infect nondividing cells (ie, they require dividing cells to be efficient). For this reason, retroviruses are generally used in ex vivo applications to transduced cultures cells (eg, axicabtagene ciloleucel; Table 2). Lentivirus vectors, like retrovirus vectors, are genome-integrating vectors and have size constraints to gene inserts. They can harbor genes of only limited size, approximately 10 kb; although most therapeutic genes of interest can be accommodated (eg, tisagenlecleucel), some are too large.4-9 To reduce the chances of generation of replication-competent lentiviruses (or replication-competent lentiviruses), a major safety concern, the nonessential viral sequences are removed. In addition, lentivirus (and retrovirus) vectors are produced using the “self-inactivating design” to reduce the risk of insertional mutagenesis.2,4-9
Adenoviruses (multiple serotypes exist), like lentiviruses, readily infect dividing and nondividing cells. They can be produced in large numbers (1012/mL), making infection of tissues in adults more efficient (may hold promise in tumor vaccines). Moreover, adenovirus vectors remain extrachromosomal, limiting transgene expression. However, there is a reduced chance of disrupting the human cellular genome.
Another limitation is that adenoviral vectors can be immunogenic, which reduces the length of time available for the expression of the gene and makes repeated administration of the vector almost impossible. In the third-generation adenoviral vectors, called “gutless adenovirus vectors,” all viral genes except the ψ and inverted terminal repeats sequences have been deleted in an attempt to ameliorate detrimental immune recognition, possibly allowing long-term expression of the gene and its product. Although gutless adenoviral vectors can package larger gene inserts of up to 37 kb, they have lower transduction efficiency than conventional adenovirus vectors.4-9
Adeno-associated vectors (numerous serotypes exist) are derived from helper virus–dependent virus. They can also infect both dividing and nondividing cells, but for best gene transduction, the dividing phase of the cell cycle is preferred. Adeno-associated vectors are capable of producing high levels of the therapeutic proteins in the target cell; unlike conventional adenoviral vectors, their expression can be maintained for years in cells with a low rate of division, such as in the liver, neurons, and skeletal muscle.1-10 The major limitation of adeno-associated vectors is size constraints, with a gene insert of about 4.7 kb and dose-dependent insertional mutagenesis (observed in mice experiments).1,10 Herpesvirus vectors (eg, talimogene laherparepvec) do not normally integrate into the host genome. However, they can be engineered to integrate with the introduction of adenoassociated vectors and inverted terminal repeats.
Not all vectors are immunogenic in humans (see Table 2). Nonviral vectors (eg, plasmid DNA) are not necessarily preferred over the viral vectors. Compared with viral vectors, nonviral plasmid DNA vectors have lower transduction efficiency. A number of methods (physical and chemical) have been explored to improve plasmid DNA efficiency, including cationic liposomes, electroporation, and magnetofection.4-9 Taken together, none of the currently available gene-transfer vectors can be referred to as “ideal” or “best” vectors for all disorders (Table 2).
Question 5
All of the following are potential obstacles of gene-vector transfer systems except?
Correct answer: D. Permanent expression.
Expert Perspective
The transgene must be delivered to the physiologically relevant target tissue or tissues, must be stably expressed, and must not interfere with the functional integrity of transduced cells.1 Major risks of integrating vectors (eg, retroviral and lentiviral vectors) arise from their potential for insertional mutagenesis (“genotoxicity”), in which the vector inserts into the DNA of a cell and disrupts a functional element of that DNA.1-11 The risk of insertional mutagenesis has been circumvented by production of safer integrated and non-integrating (eg, adeno-associated in most cases) vectors, and the risk of an immune response has been reduced through the use of adjuvant immunosuppression (eg, glucocorticoids).1,4-9
Gene silencing implies gradual loss of gene expression without evidence of immune response. This, along with phenotoxicity (ectopic expression of transgene), horizontal and vertical transmission (household contact seropositivity and offspring positivity, respectively) is a theoretical concern associated with certain viral vectors (eg, adeno-associated vector). Ideally, in inherited disorders, a transduced gene capable of expressing the therapeutic product for the patient’s entire life is desired (ie, permanent reversal of the disease phenotype).1
Question 6
Which statement about gene-editing systems is correct?
Correct answer: C. Off-target toxicity is a serious concern with these systems.
Expert Perspective
Unlike gene-vector delivery systems, gene-editing systems involve directly altering the specific DNA sequence of target cells (“gene scissors”). In general, the new sequence is introduced using a template, which is delivered into the target cell together with the endonuclease enzyme.3-4,6-11 For these gene-editing systems to be efficient and safe, the precision of target DNA recognition, double-strand DNA cleavage, and its repair must be extremely specific, with exact in-frame changes and without causing off-target toxicity elsewhere in the genome. These technologies have the ability to insert, delete, or alter the genome at specific locations.
In addition to concerns for off-target toxicity, an inadvertent immune response charged toward a previously absent protein is also a legitimate concern (eg, anti–gene product antibody). In theory, such targeted platforms could allow the corrected target genes to be expressed under the control of their native regulatory elements and thus eliminate the risk of insertional mutagenesis. For each of these systems to effectively enter cells of interest and perform their function, efficient and safe delivery technologies are being investigated. ■
The authors would like to extend their gratitude to and acknowledge Dr. Helen E. Heslop for her critical review of this article.
DISCLOSURE: Dr. Abutalib is an advisor for AstraZeneca and Partner Therapeutics. Dr. Ibrahim reported no conflicts of interest.
REFERENCES
1. High KA, Roncarolo MG: Gene therapy. N Engl J Med 381:455-464, 2019.
4. Gonçalves GAR, Paiva RMA: Gene therapy: Advances, challenges and perspectives. Einstein (Sao Paulo) 15:369-375, 2017.
5. Collins M, Thrasher A: Gene therapy: Progress and predictions. Proc Biol Sci 282:20143003, 2015.
7. Larochelle A, Dunbar CE, Tisdale J: Gene therapy for hematologic disorders, in Greer JP, Arber DA, Glader B, et al: Wintrobe’s Clinical Hematology, 13th ed, pp 1492-1522. Beijing, Wolters Kluwer Health, 2014.
8. Fung H, Gerson SL: Gene therapy in hematopoietic cell transplants, in Lazarus HM, Gale RP, Keating A, et al: Hematopoietic Cell Transplants: Concept, Controversies and Future Directions, pp 649-656. Cambridge, UK; Cambridge University Press, 2017.
10. Chandler RJ, et al: Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest 125:870-880, 2015.
11. Antony JS, et al: CRISPR/Cas9 system: A promising technology for the treatment of inherited and neoplastic hematological diseases. Adv Cell Gene Ther 1(e10):1-9, 2018.

