 Data presented at the ASCO Annual Meeting this year on the management of locoregional lung cancer present a mixed picture, with some advances and some disappointments, according to H. Jack West, MD, of the Swedish Cancer Institute in Seattle, who reviewed studies in this area at the Best of ASCO Seattle meeting.
Data presented at the ASCO Annual Meeting this year on the management of locoregional lung cancer present a mixed picture, with some advances and some disappointments, according to H. Jack West, MD, of the Swedish Cancer Institute in Seattle, who reviewed studies in this area at the Best of ASCO Seattle meeting.
The randomized phase II TREAT trial demonstrated that when it comes to adjuvant therapy for non–small cell lung cancer (NSCLC), the doublet of cisplatin and pemetrexed (Alimta) is more feasible to administer than the doublet of cisplatin and vinorelbine.1
Among the 132 patients studied, the rate of clinical feasibility (defined as no deaths due to cancer, toxicity, or comorbidities; no patient early withdrawals; and no dose-limiting toxicities) was higher with cisplatin/pemetrexed (95.5% vs 75.4%, P = .001; Table 1). The mean duration of therapy was similar (11.2 vs 9.9 weeks). Dose delays and interruptions were about twice as common in the cisplatin/vinorelbine arm, solely due to adjustments in vinorelbine.
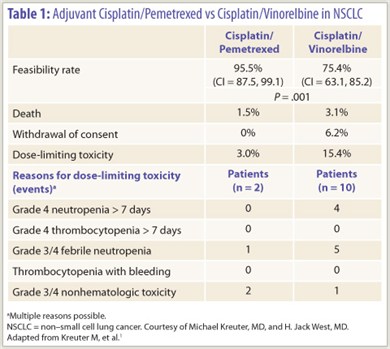 Generalizability of the trial is limited, according to Dr. West. “This is a younger, cherry-picked, fitter population than at least the people who walk into my office on average,” he noted. Also, the majority were male, and 38% had stage IB disease (a group for whom chemotherapy has debatable benefit).
Generalizability of the trial is limited, according to Dr. West. “This is a younger, cherry-picked, fitter population than at least the people who walk into my office on average,” he noted. Also, the majority were male, and 38% had stage IB disease (a group for whom chemotherapy has debatable benefit).
“Adjuvant cisplatin-based chemotherapy is still standard of care, at least for stage II and IIIA resected NSCLC, and arguably for selected stage IB patients,” Dr. West said. “The [existing] evidence is strongest for cisplatin and vinorelbine,…but the reality is that it is truly challenging to administer. So despite the lack of randomized data in the adjuvant setting, cisplatin/pemetrexed is clearly more feasible per the TREAT trial.”
That said, there are several other reasonable options, such as cisplatin/gemcitabine. “And in truth, it needs to be an individualized decision with your judgment, working with the patient in front of you,” he maintained.
Proteomic Profile in Cancer Detection
 In patients with lung nodules that are clinically suspicious for stage I cancer, a new serum proteomic profile is not helpful for determining which are indeed cancer and which are simply benign lesions, finds the phase II American College of Surgeons Oncology Group (ACOSOG) Z4031 trial.2
In patients with lung nodules that are clinically suspicious for stage I cancer, a new serum proteomic profile is not helpful for determining which are indeed cancer and which are simply benign lesions, finds the phase II American College of Surgeons Oncology Group (ACOSOG) Z4031 trial.2
Serum was collected before and 60 and 90 days after surgery for resection of the nodules in 913 patients and analyzed by mass spectrometry. Pathology showed that 79% of the patients had NSCLC.
Mass spectrometry failed to identify a sufficient number of high-signal proteins, resulting in low sensitivity of the profile. In the validation set, the profile failed to discriminate significantly any-histology NSCLC vs benign lesions; squamous NSCLC vs benign lesions; adenocarcinoma NSCLC vs benign lesions; or squamous vs adenocarcinoma NSCLC.
“The implications today: No need to go back and start sending off [serum] for this. It’s not yet ready for use,” Dr. West commented. “But it’s still an admirable goal, and I hope and expect we’ll see more efforts along these lines, hopefully more successfully as our technology improves.”
Results have been more encouraging in another recent study that also used mass spectrometry proteomics, but this time working with a predefined biomarker profile generated using prospectively collected data from tumor tissue and cell lines.3 Results suggested that this profile does discriminate NSCLC from controls. Moreover, it works similarly well in patients who had never smoked.
“This is still early, it’s not yet available, and it needs more validation,” Dr. West cautioned. “But it at least should give a glimpse that this is not pie-in-the-sky work and that we may still succeed.”
Disease-free Survival as Surrogate for Overall Survival
In a development that may help expedite clinical trials, new data show that disease-free survival can serve as an earlier surrogate endpoint for overall survival in patients who undergo lung cancer resection and receive adjuvant chemotherapy.4
Investigators conducted a pair of meta-analyses using individual patient data. One analysis used data from 5,379 patients in 17 trials testing surgery with vs without adjuvant chemotherapy (median follow-up, 5.7 years). The other analysis used data from 2,247 patients in 7 trials testing surgery plus radiation therapy, with vs without adjuvant chemotherapy (median follow-up, 6.4 years).
In the first meta-analysis, investigators found a strong correlation between disease-free survival and overall survival at the patient level (r = 0.91) and the trial level (R = 0.96). Similarly, in the second meta-analysis, there was a strong correlation between disease-free survival and overall survival at the patient level (r = 0.93) and the trial level (R = 0.99).
 Additionally, in the analysis looking at surgery plus chemotherapy, with vs without radiation therapy, about 80% of all recurrences occurred within the first 3 postoperative years.
Additionally, in the analysis looking at surgery plus chemotherapy, with vs without radiation therapy, about 80% of all recurrences occurred within the first 3 postoperative years.
“So [the investigators] propose that disease-free survival can be used as a surrogate for overall survival in adjuvant chemotherapy for lung cancer, potentially as the primary endpoint,” Dr. West noted. But he also concurred with them that new analyses will be needed to see if the same holds true with targeted therapies. “It would be a mistake to generalize these results,” he said.
“My own caveat on this is that I am not sure that the results will hold as true in an era today of effective therapies,” he commented. “Today’s postrecurrence treatments work better, and patients are living longer, which may weaken the correlation between disease-free and overall survival and necessitate longer follow-up.” ■
Disclosure: Dr. West reported no potential conflicts of interest.
SIDEBAR: Searching for More Efficacious, Less Toxic Adjuvant Chemotherapy for NSCLC
References
1. Kreuter M, Vansteenkiste JF, Fischer JR, et al: Randomized phase II trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed (CPx) versus cisplatin and vinorelbine (CVb): TREAT. 2011 ASCO Annual Meeting. Abstract 7002. Presented June 7, 2011.
2. Harpole D, Ballman KV, Oberg AL, et al: Proteomic analysis for detection of NSCLC: Results of ACOSOG Z4031. 2011 ASCO Annual Meeting. Abstract 7003. Presented June 7, 2011.
3. Birse C, Laiger R, Bruce R, et al: Serum biomarker panel detects lung cancer in never smokers. American Association for Cancer Research 102nd Annual Meeting. Abstract 2813. Presented April 4, 2011.
4. Michiels S, Mauguen A, Fisher D, et al: Evaluation of disease-free survival as surrogate endpoint for overall survival using two individual patient data meta-analyses of adjuvant chemotherapy in operable non-small cell lung cancer. 2011 ASCO Annual Meeting. Abstract 7004. Presented June 7, 2011.

