A number of different strategies for combining chemotherapy and radiation therapy have been evaluated in the effort to improve survival in patients with high-risk medulloblastoma. In a trial (POG 9031) reported in Journal of Clinical Oncology, Nancy J. Tarbell, MD, of Massachusetts General Hospital, and colleagues in the Pediatric Oncology Group assessed the effects of chemotherapy before vs after radiotherapy following surgery in children with high-risk medulloblastoma.1 They found no difference in 5-year event-free survival with the two strategies.
Study Details
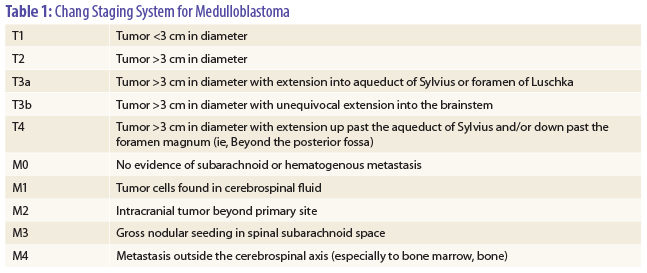
In the trial, 224 patients aged 3 to 21 years with high-risk medulloblastoma were randomized after surgery to chemotherapy with three cycles of cisplatin at 90 mg/m2 and etoposide at 150 mg/m2 over 7 weeks followed by radiotherapy (n = 112) or radiotherapy followed by the same chemotherapy (n = 112). Patients were considered high risk if they had M1–4 disease by modified Chang staging classification (see Table 1 on page 18), exhibited T3b/T4 disease at time of surgery, or had greater than 1.5 cm3 of residual tumor after surgery.
Radiotherapy consisted of craniospinal irradiation of 35.2 to 44.0 Gy and posterior fossa boost of 53.2 to 54.4 Gy. All patients received consolidation chemotherapy consisting of vincristine and cyclophosphamide for 28 weeks. The median age was 7.8 years (range, 3.0–21.4 years). The chemotherapy-first and radiotherapy-first groups were balanced for sex (55% and 62% male), race (79% and 74% white), M stage (52% M0 in both groups), and T stage (3b or 4 in 73% and 70%); 40 patients in the chemotherapy-first group and 32 in the radiotherapy-first group were T3b/T4 M0 with no residual disease.
Survival Outcomes
Median follow-up was 6.4 years. Five-year event-free survival was 66.0% in the chemotherapy-first group and 70.0% in the radiotherapy-first group (P = .54), and 5-year overall survival in the two groups was 73.1% and 76.1% (P = .47). There was no evidence of a difference in event-free survival between treatments after stratifying for stage.
In patients with M0 and residual disease after surgery, 5-year event-free survival was 59.6% in the chemotherapy-first group and 65.1% in the radiotherapy-first group (P = .40). In patients with M+ and residual disease, 5-year event-free survival was 51.2% and 64.0% (P = .20). Objective response rates in evaluable patients were 66% in the chemotherapy-first group and 86% in the radiotherapy-first group (P = .01). There was no significant difference in 5-year event-free survival between patients in the chemotherapy-first group who achieved response and those who did not (73% vs 56%, P = .1).
Patients with M0-1 disease received a median of 35.2 Gy craniospinal irradiation and 53.2 Gy to the posterior fossa, and patients with M2-3 disease received a median of 40 Gy craniospinal irradiation and 54.4 Gy to the posterior fossa, with these regimens not differing according to treatment group. The average overall radiotherapy treatment times were 46.3 days (standard deviation, 10.51 days) in the chemotherapy-first group and 44.8 days (standard deviation, 8.98 days) in the radiotherapy-first group. Twenty-two patients in the chemotherapy-first group (20%) and 11 patients in the radiotherapy-first group (10%) had more than 50 treatment days, with the difference approaching significance (P = .06).
M Stage Predicts Outcome
On regression analysis, factors such as sex, race, and regimen were not significant predictors of event-free or overall survival, with M stage (see Table 1) being the only factor associated with outcome. The 5-year event-free survival rates were 22% for M4 vs 70% for patients with M0, M1, M2, or M3 disease (P < .001). For M4 patients, the relative risk for progression or relapse was four times higher (95% confidence interval [CI] = 1.9–8.9) vs patients with M0, M1, M2, or M3 disease, and the relative risk for death was 4.3 times higher (95% CI = 2.0–9.4 times).
With regard to acute toxicity, there were more episodes of thrombocytopenia in the radiotherapy-first group, but toxicity was otherwise similar in the two groups. Treatment-induced disease included myelodysplastic syndrome in two patients and ossifying fibroma in one.
The investigators concluded, “The [event-free and overall survival] reported in this study compare favorably with other trials examining high-risk medulloblastoma. Preirradiation chemotherapy fails to confer a survival advantage over radiation before chemotherapy.” ■
Disclosure: Dr. Tarbell reported no potential conflicts of interest.
Reference
1. Tarbell NJ, Friedman H, Polkinghorn WR, et al: High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. July 15, 2013.