
Syed Ali Abutalib, MD

David L. Porter, MD
On October 18, 2017, the U.S. Food and Drug Administration (FDA) granted approval to axicabtagene ciloleucel for the treatment of adult patients with relapsed or refractory large B-cell lymphoma. Specifically, this treatment can be used after two or more lines of systemic therapy for diffuse large B-cell lymphoma (DLBCL)–not otherwise specified, primary mediastinal large B-cell lymphoma, high-grade B-cell lymphoma, and DLBCL arising from follicular lymphoma1 (Table 12,3).
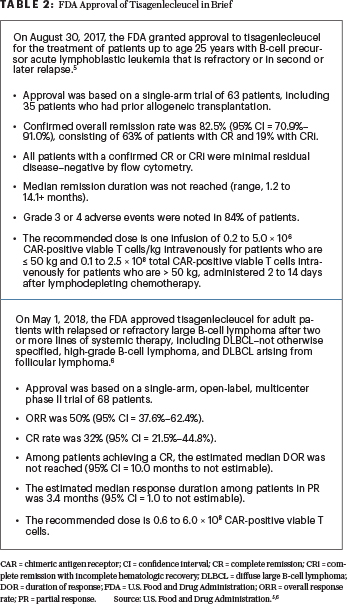
Data are now emerging on the use of commercial chimeric antigen receptor (CAR) T cells in the treatment of relapsed or refractory large B-cell lymphoma.4 Here, we review selected presentations involving FDA-approved CAR T-cell therapy products for the treatment of DLBCL presented at the 2019 ASCO Annual Meeting. During this meeting (as opposed to the 2018 American Society of Hematology Meeting & Exposition), the presentations on tisagenlecleucel were limited; however, it is important to emphasize the fact that tisagenlecleucel is also approved for the treatment of patients with relapsed or refractory DLBCL.5,6 Tisagenlecleucel is approved for two CD19-positive hematologic conditions (Table 2).
Outcomes After Immunotherapy
ABSTRACT 7517: Outcomes in relapsed or refractory large B-cell lymphoma progressing after commercialization of axicabtagene ciloleucel: Retrospective analysis from the U.S. Lymphoma CAR-T Consortium.7
Background: The outcomes for patients who fail to respond to or who experience disease progression after axicabtagene ciloleucel are unknown.
Key Findings: Of a total of 274 patients with a median follow-up of 14 months, 116 had progressive disease as of February 2019. Following relapses, 60% (n = 61) underwent biopsy, and 70% (n = 43) had CD19 expression measured. Of these 43 patients, 30% (n = 13) were CD19-negative by immunohistochemistry (3 of 13), flow (2 of 13), or both (8 of 13). A total of 70% of patients received salvage therapy for progressive disease. The median number of salvage therapies was one (range, 0–4).
A total of 19 patients relapsed up to 3 months after treatment with axicabtagene ciloleucel and did not receive therapy, with a median overall survival of 17 days (95% confidence interval = 7–49 days); 33 patients relapsed up to 3 months after treatment and received therapy, with a median overall survival of 114 days (95% lower confidence limit [LCL] = 82 days). In contrast, 30 patients who relapsed more than 3 months after treatment with axicabtagene ciloleucel and received therapy had an estimated median overall survival of more than 220 days (95% LCL = 81 days).
Clinical Implications: Axicabtagene ciloleucel may have dramatic activity in some patients with relapsed or refractory lymphoma, but sustained remissions are anticipated in just about 40% of patients. Therefore, the majority of patients will fail to respond or will experience disease progression. Loss of CD19 antigen may be one mechanism of resistance, which was noted in 30% of patients in this study. Patients whose disease progressed soon after treatment with axicabtagene ciloleucel tended to have poor outcomes. Enhancing activity of CAR T cells, preventing CD19 antigen–negative relapses, and developing novel strategies to treat patients who experience disease progression or fail to respond to treatment are critically important.

ABSTRACT 7558: Preliminary results of earlier steroid use with axicabtagene ciloleucel in patients with relapsed or refractory large B-cell lymphoma (ClinicalTrials.gov identifier NCT02348216)8
Background: Axicabtagene ciloleucel treatment has been associated with a high incidence of cytokine-release syndrome and neurologic toxicity. In ZUMA-12, grade ≥ 3 cytokine-release syndrome and neurologic events occurred in 11% and 32% of patients, respectively; 26% of patients received steroids, and 43% received tocilizumab.2 A safety expansion cohort was added to evaluate the effect of earlier steroid use on the rates of these adverse events. Patients in this cohort received early steroid intervention, starting at grade 1 cytokine-release syndrome or neurologic events when no improvement was observed after 3 days of supportive care. There is a hypothetical concern that early use of steroids may attenuate the efficacy of CAR T cells.
Delayed recovery of CD4-positive T cells beyond 1 year in 33% of patients highlights the need for continued vigilance for opportunistic infections.— Syed Ali Abutalib, MD, and David L. Porter, MD
Tweet this quote
Key Findings: As of September 2018, 21 of 40 planned patients received axicabtagene ciloleucel, with a minimum follow-up of 1 month (median, 2.6 months). A total of 76% of patients received steroids, and 81% received tocilizumab. Grade 1 and 2 neurologic events occurred in 38% and 5% of patients, respectively. No patient had grade ≥ 3 cytokine-release syndrome, 33% of patients had grade 1 cytokine-release syndrome, and 67% had grade 2 cytokine-release syndrome. There were no deaths due to adverse events; one patient died due to disease progression.
Clinical Implications: Early use of steroids may help to manage severe cytokine-release syndrome and neurologic events by potentially reducing their incidence in patients treated with axicabtagene ciloleucel without affecting response rates. Presently, follow-up is insufficient to determine whether earlier use of steroids will impact CAR T-cell activity.
ZUMA-1 and ZUMA-9 Trials
ABSTRACT 7555: Outcomes of patients ≥ 65 years of age (n = 27) in ZUMA-12, a pivotal phase I/II study of axicabtagene ciloleucel in refractory large B-cell lymphoma (NCT02348216)9
Background: Data regarding the outcomes after treatment with the commercial product are beginning to emerge.7 Typically, older patients respond less well and have significantly more toxicity to conventional cancer therapies than younger patients. The impact of age on response and toxicity to CAR T cells is not well defined.
Key Findings: Of about 108 recipients of axicabtagene ciloleucel treated on the ZUMA-1 study2, 24 were aged 65 years or older. The median follow-up was 27.1 months. Response rates and toxicity for older and younger patients were similar. The overall response rates for patients aged 65 years and older (n = 24) and younger than age 65 (n = 77) were 92% and 81%, with complete response rates of 75% and 53%, respectively. Furthermore, ongoing complete remission rates were 42% and 35%, respectively.
Most patients experienced grade ≥ 3 adverse events (100% of patients ≥ 65 years; 98% of patients < 65 years), and 4% of each group died due to adverse events, as previously reported. Grade ≥ 3 neurologic events and cytokine-release syndrome occurred in 44% vs 28% and 7% vs 12% of patients at least 65 years old vs up to age 65, respectively.
Clinical Implications: The 2-year follow-up of ZUMA-12 demonstrated that axicabtagene ciloleucel treatment may induce high rates of durable responses with a manageable safety profile in older adults with refractory large B-cell lymphoma, with an overall survival rate of 54%, compared with 49% for younger patients. Therefore, an age limit of 65 years alone should not exclude patients from CAR T-cell therapy. Additional detailed data will be necessary to determine whether patients of even older ages have similar outcomes.
ABSTRACT 7545: Hematopoietic recovery and immune reconstitution after axicabtagene ciloleucel in patients with relapsed or refractory large B-cell lymphoma10
Background: Cytopenias after CAR T-cell therapies appear to be common, but the frequency, duration, and severity are not yet well described or understood. Of more than 100 patients treated for relapsed or refractory lymphoma on ZUMA-12 and ZUMA-9 (expanded-access study [NCT03153462]) studies, there were data and samples available for analysis for 31 patients.
Key Findings: Of these 31 patients, grade ≥ 3 cytopenias were observed in 15 patients (48%) at day +30, represented by neutropenia in 9 patients, anemia in 5 patients, and thrombocytopenia in 13 patients. No significant association was observed between day +30 grade ≥ 3 cytopenia and subsequent grade ≥ 3 infections (27% vs 31%, P = 1.0), need for growth factor support (both 100%), red blood cell transfusions, or diagnosis of myelodysplastic syndromes. At a median follow-up of 2.7 years, a trend for a higher 3-year progression-free survival was observed for patients with day +30 grade ≥ 3 cytopenia (63% vs 35%, P = .18). Among patients with ongoing response at most recent follow-up, resolution of grade ≥ 3 cytopenia was observed in 75% of patients at 1 year and 86% at 2 years.
Clinical Implications: Cytopenias are common after treatment with axicabtagene ciloleucel (and other CAR T-cell therapies). Not unexpectedly, patients with day +30 grade ≥ 3 cytopenia had a significantly higher use of intravenous immunoglobulins (67% vs 25%, P = .03) and platelet transfusion (73% vs 25%, P = .01). The possible association of day +30 grade ≥ 3 cytopenia with progression-free survival suggests cytopenias could be an indication of CAR T-cell activity, although the mechanisms of cytopenias are poorly understood. It is notable that grade > 3 cytopenias resolve in most patients by 1 year. In patients with ongoing responses, CD19 recovery was seen in just 25% of patients, suggesting possible persistence of CAR T cells, though these data were not presented. Furthermore, delayed recovery of CD4-positive T cells beyond 1 year in 33% of patients highlights the need for continued vigilance for opportunistic infections. The best strategies to manage prolonged cytopenias and delayed immune reconstitution after treatment with CAR T cells will need prospective assessments to best inform optimal clinical care. ■
Dr. Abutalib is Associate Director, Hematology and Cellular Therapy Program; Director, Clinical Apheresis Program; Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Roseland Franklin University of Medicine and Science; Founder and Co-Editor, Advances in Cell and Gene Therapy. Dr. Porter is Director, Cell Therapy and Transplantation, and Jodi Fisher Horowitz Professor in Leukemia Care Excellence, Abramson Cancer Center and Perelman School of Medicine, University of Pennsylvania, Philadelphia.
DISCLOSURE: Dr. Abutalib is an advisor for AstraZeneca. Dr. Porter has an immediate family member who has been employed by Genentech/Roche; has an immediate family member who has held stock or other ownership interests in Genentech/Roche; has served in a consulting or advisory role for Bellicum Pharmaceuticals, Gerson Lehrman Group, Glenmark, Incyte, Kite Pharma, and Novartis; holds a patent that is licensed to Novartis by the University of Pennsylvania; has been reimbursed for travel, accommodations, and other expenses by Immunovative Therapies, Kite Pharma, and Novartis; and has been a Member and Chair of the National Marrow Donor Program’s Board of Directors and a member of the American Board of Internal Medicine’s Hematology Exam Writing Committee.
REFERENCES
1. U.S. Food and Drug Administration: FDA approves axicabtagene ciloleucel for large B-cell lymphoma. Available at www.fda.gov/drugs/informationondrugs/approveddrugs/ucm581296.htm. Accessed August 8, 2019.
2. Locke FL, Ghobadi A, Jacobson CA, et al: Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase I/II trial. Lancet Oncol 20:31-42, 2019.
3. Lee DW, Gardner R, Porter DL, et al: Current concepts in the diagnosis and management of cytokine release syndrome. Blood 124:188-195, 2014.
4. Nastoupil LJ, Jain MD, Spiegel JY, et al: Axicabtagene ciloleucel CD19 chimeric antigen receptor T-cell therapy for relapsed/refractory large B-cell lymphoma: Real world experience. 2018 ASH Annual Meeting & Exposition. Abstract 91.
5. U.S. Food and Drug Administration: FDA approves tisagenlecleucel for B-cell ALL and tocilizumab for cytokine release syndrome. Available at www.fda.gov/drugs/informationondrugs/approveddrugs/ucm574154.htm. Accessed August 8, 2019.
6. U.S. Food and Drug Administration: FDA approves tisagenlecleucel for adults with relapsed or refractory large B-cell lymphoma. Available at www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm606540.htm. Accessed August 8, 2019.
7. Spiegel JY, Dahiya S, Jain MD, et al: Outcomes in large B-cell lymphoma progressing after axicabtagene ciloleucel: Results from the U.S. Lymphoma CAR-T Consortium. 2019 ASCO Annual Meeting. Abstract 7517. Presented June 3, 2019.
8. Topp MS, van Meerten T, Wermke M, et al: Preliminary results of earlier steroid use with axicabtagene ciloleucel in patients with relapsed/refractory large B-cell lymphoma. 2019 ASCO Annual Meeting. Abstract 7558. Presented June 3, 2019.
9. Neelapu SS, Jacobson CA, Oluwole OO, et al: Outcomes of patients ≥ 65 years of age in ZUMA-1, a pivotal phase I/II study of axicabtagene ciloleucel in refractory large B-cell lymphoma. 2019 ASCO Annual Meeting. Abstract 7555. Presented June 3, 2019.
10. Strati P, Adkins S, Nastoupil LJ, et al: Hematopoietic recovery and immune reconstitution after axi-cel CAR T-cell therapy in patients with relapsed/refractory large B-cell lymphoma. 2019 ASCO Annual Meeting. Abstract 7545. Presented June 3, 2019.

