Long-term cardiac safety and efficacy have been confirmed for pertuzumab plus trastuzumab in patients with early breast cancer, in an update of the phase II BERENICE trial reported at the 2021 ESMO Breast Cancer Virtual Congress.1
Chau T. Dang, MD, of the Breast Medicine Service at Memorial Sloan Kettering Cancer Center, New York, reported no new cardiac safety issues and a 5-year event-free survival rate of around 90% with neoadjuvant/adjuvant dual anti-HER2 blockade plus anthracycline-containing regimens in patients with early HER2-positive breast cancer.

Chau T. Dang, MD
“These data support the use of dual anti-HER2 blockade with pertuzumab/trastuzumab-based regimens, including the combination with dose-dense anthracycline-based chemotherapy, across the neoadjuvant and adjuvant treatment settings for the complete management of HER2-positive early breast cancer,” Dr. Dang commented.
About BERENICE
BERENICE was a multicenter, open-label, noncomparative phase II trial designed to establish the cardiac safety of neoadjuvant/adjuvant pertuzumab/trastuzumab with anthracycline-containing chemotherapy in patients with stage IIA to III HER2-positive early breast cancer (< 2 cm or > 5 mm and node-positive). All patients had a baseline left-ventricular ejection fraction of at least 55%.
Patients were allocated to the following regimens:
- Dose-dense doxorubicin plus cyclophosphamide every 2 weeks for 4 cycles followed by weekly paclitaxel for 12 cycles (cohort A)
- Fluorouracil, epirubicin, and cyclophosphamide (FEC) every 3 weeks for 4 cycles plus docetaxel every 3 weeks for 4 cycles (cohort B)
- Pertuzumab/trastuzumab in both arms, initiated with the taxane and continued after surgery for a total of 17 cycles.
Cardiac Safety
As previously reported, the primary analysis showed a low incidence of cardiac events during the neoadjuvant period and high rates of total pathologic complete response.2 Two patients in the dose-dense arm (cohort A) experienced a class III or IV heart failure event, and one patient in that arm experienced two such events. No cardiac events were observed in the FEC arm (cohort B). Declines in left-ventricular ejection fraction were observed in 6.5% of cohort A and 2.0% of cohort B. Pathologic complete response rates were approximately 61% in each arm.
Updated 5-Year Outcomes
Dr. Dang reported the 5-year outcomes from an end-of-study analysis, including additional safety and efficacy data, after a median follow-up of 64.5 months.
“No new safety concerns arose during long-term follow-up, with a low incidence of cardiac events (Table 1), drug-related grade ≥ 3 adverse events, and serious adverse events in both cohorts,” she said.
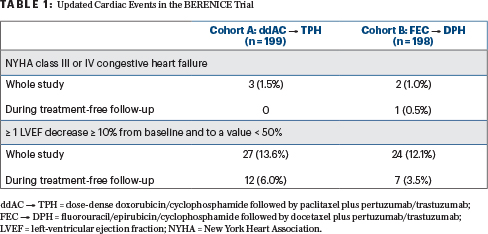
As for other safety parameters, for cohorts A and B, respectively, grade ≥ 3 adverse events were seen in 1.0% and 2.5%; serious adverse events were noted in 1.5% and 3.5%; non–breast-related second primary malignancies were seen in 0% and 3.0%; and deaths were reported in 3.5% and 6.5%. The most common cause of death in both cohorts was disease progression.
Event-free survival rates at 5 years for cohorts A and B, respectively, were 90.8% and 89.2%. As previously reported, at 3 years, they were 93.6% and 90.8%. Overall survival rates at 5 years were 96.1% and 93.8%, respectively.
DISCLOSURE: Dr. Dang has received honoraria from and has served as a consultant or advisor to Daiichi Sankyo, Evicore Healthcare, Genentech, Lilly, Novartis, Pfizer, Puma Biotechnology, Roche, Pfizer, Novartis, and Evicore Healthcare; and has received support from Roche for third-party editorial assistance from Alison McGonagle, PhD, of Health Interactions.
REFERENCES
1. Dang C, Ewer MS, Delaloge S, et al: Pertuzumab/trastuzumab in early stage HER2-positive breast cancer: 5-year and final analysis of the BERENICE trial. 2021 ESMO Breast Cancer Virtual Congress. Abstract 43O. Presented May 7, 2021.
2. Swain SM, Ewer MS, Viale G, et al: Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann Oncol 29:646-653, 2018.

