Don’t expect metastatic lung cancer to be cured any time soon, says Paul A. Bunn, Jr, MD, Professor and James Dudley Chair in Cancer Research at the University of Colorado School of Medicine, Denver.
“You have to be disease-free for some length of time in order to be cured, which is our goal,” he said. Unfortunately, by 2 years, the majority of patients with stage IV lung cancer show disease progression, in spite of whatever promising experimental agent they may have received.
“Molecular therapies have improved the quality and quantity of life, but they are unlikely to cure non–small cell lung cancer patients when used in any stage of disease,” Dr. Bunn said in a keynote lecture during the 15th Annual International Lung Cancer Congress in Huntington Beach, California.
Nevertheless, he acknowledged that the identification of driver mutations is a step in the right direction. Sequential tyrosine kinase inhibitors and novel combinations appear to be keeping patients alive longer, and immunotherapy is promising, albeit unproven, he said.
Phase III Trials of Targeted Agents
Dr. Bunn ran through a list of molecularly targeted agents and their outcomes in unselected phase III trial populations:
Necitumumab plus gemcitabine/cisplatin in squamous cell non–small cell lung cancer: median overall survival improvement of 1.6 months (hazard ratio [HR] = 0.84) but no improvement in non–squamous cell subsets.1 Necitumumab is an antibody against the epidermal growth factor receptor (EGFR).
Ramucirumab (Cyramza) plus docetaxel, in the second line: median overall survival improvement of 1.4 months (HR = 0.86).2 Ramucirumab is an antibody against vascular endothelial growth factor (VEGF) receptor 2.
Nintedanib plus docetaxel: median overall survival improvement of 2.3 months in patients with non–squamous cell histology (HR = 0.83) but no improvement in the overall population.3 Nintedanib is a small-molecule multi–tyrosine kinase inhibitor.
Discussing these studies, Dr. Bunn made the following points: (1) it is necessary to determine in which subsets the biologic effect of these agents is seen, (2) evaluating drugs in the second-line setting in combination with docetaxel is not the best measure of their worth, (3) at around 18 months, few patients remain disease-free, regardless of statistically significant benefits that emerge in trials, and (4) about 20% of patients are still alive at 2 years.
“It doesn’t take a genius to know that after many randomized trials in unselected patients, which show only tiny advantages or negative outcomes, we are not going to cure patients by conducting this type of trial,” he said. “Not that they should not be done, but we won’t cure anyone.”
Targeting Driver Mutations
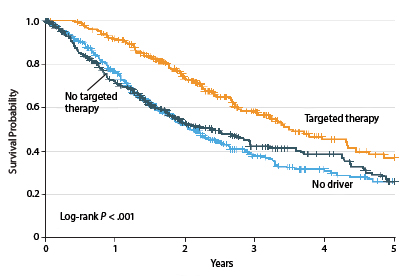
The Lung Cancer Mutation Consortium recently identified driver mutations in 64% of 1,007 patients with advanced adenocarcinoma.4 These were primarily in KRAS (25%), EGFR (sensitizing, 17%), and ALK (8%). By matching the individual genotype with a targeted treatment, outcomes were improved (Fig. 1).
Median overall survival was 3.5 years for the 260 patients with an oncogenic driver and genotype-directed therapy, compared with 2.4 years for the 318 patients with a driver mutation but no targeted therapy, and approximately 2 years for patients lacking a driver mutation.
“A median overall survival of 3.5 years in stage IV disease, with drugs that are better tolerated than chemotherapy—one could say this is a shift in the treatment paradigm,” Dr. Bunn said.
“Clearly, this approach has had a major impact for patients with a driver mutation,” he noted. “On the other hand, the survival curves are still heading toward zero.”
The study also showed that some genes can be more effectively targeted than others. Patients with multiple oncogenic drivers, and those with HER2 and KRAS mutations, had worse outcomes than patient with abnormalities of ALK, BRAF, and EGFR.
Numerous randomized trials have confirmed that when patients with mutations receive the appropriate EGFR tyrosine kinase inhibitor, response rates are higher and progression-free survival is better than with chemotherapy. In terms of overall survival, however, the order in which they receive these drugs vs chemotherapy is inconsequential. From the patient’s perspective, the oral tyrosine kinase inhibitor is preferred, he said.
Unfortunately, resistance to targeted agents is universal. Tumor cells escape from the initial tyrosine kinase inhibitor through a secondary gatekeeper mutation or through activation of another signaling pathway. Third-generation tyrosine kinase inhibitors may help in this regard: those that do not bind to the wild-type receptor do bind to the EGFR T790M receptor gatekeeping mutation.
“With these agents, such as AZD9291, we have seen very high response rates and very long durations of response, with median progression-free survival exceeding 2 years,” he indicated. “Nonetheless, every patient in these studies is still relapsing.”
Dr. Bunn maintained that using tyrosine kinase inhibitors as adjuvant treatment would probably have little effect on long-term outcomes. When this has been tried, patients have disease progression once the drug is stopped, and none are cured.
“In my opinion, there’s not a whit of evidence that adjuvant treatment is better than waiting until the disease recurs, and then putting the patient on the [tyrosine kinase inhibitor],” he said. “Keeping patients on these drugs for 10 years is not a real treat for them.”
He added, “I don’t think we can cure patients with molecular therapy at the moment, even in the early stage of their disease.”
The same pattern holds for patients with the ALK translocation. While third-generation ALK inhibitors do provide a “reasonable” time to progression, by 2 years—once again—the majority of patients have had disease progression, he noted.
Immunotherapy With Checkpoint Inhibitors
Could immunotherapy be the answer? Approximately 20% of patients with advanced disease respond to the immune checkpoint inhibitors, and response is not histology-dependent. Some responses are quite durable, and this has generated tremendous excitement in the lung cancer field.
This exuberance may be premature, Dr. Bunn maintained. “There’s a phase III randomized trial comparing nivolumab to docetaxel. The media are treating this as if the trial has been completed and the results are positive,” he said.
He pointed out that the survival curves for nivolumab are virtually the same as for ramucirumab and nintedanib. “Just because 20% of patients are alive at 2 years, to me, does not mean that without patient selection immune checkpoint inhibition will be a winner,” he commented. “Like everything else, selection with a biomarker, in the long run, will be the answer—not just giving these agents to all patients who come through the door.”
Currently, the optimal biomarker is not clear. PD-L1 expression is the best candidate so far, he said, but there are issues in measuring it, determining thresholds, dealing with variability in testing and reproducibility, and so forth. “At least it’s a start,” Dr. Bunn acknowledged.
“With immunotherapy, time will tell,” he concluded. “Let’s not yet assume these agents have the ability to cure. We just hope they will.” ■
Disclosure: Dr. Bunn has consulted for Amgen, AstraZeneca, Bayer, Biodesix, Boehringer-Ingelheim, Bristol-Myers Squibb, Celgene, Daiichi-Sankyo, Eli Lilly, GSK, Merck, Novartis, OSI/Genentech/Roche, and Sanofi-Aventis.
References
1. Thatcher N, Hirsch FR, Szczesna A, et al: A randomized, multicenter, open-label, phase III study of gemcitabine-cisplatin chemotherapy plus necitumumab versus GC alone in the first-line treatment of patients with stage IV squamous non-small cell lung cancer. ASCO Annual Meeting. Abstract 8008. Presented June 2, 2014.
2. Garon EB, Ciuleanu TE, Arrieta O, et al: Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet. June 2, 2014 (early release online).
3. Reck M, Kaiser R, Mellemgaard A, et al: Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): A phase 3, double-blind, randomised controlled trial. Lancet Oncol 15:143-155, 2014.
4. Kris MG, Johnson BE, Berry LD, et al: Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 311:1998-2006, 2014.