In the Clinic provides overviews of novel oncology agents, addressing indications, mechanisms, administration recommendations, safety profiles, and other essential information needed for the appropriate clinical use of these drugs.
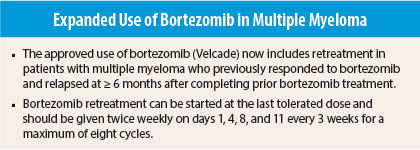
On August 8, 2014, the approved use of bortezomib (Velcade) in multiple myeloma was expanded to include bortezomib retreatment in patients who have previously responded to bortezomib and who relapsed at least 6 months after completing prior bortezomib treatment.1 Retreatment may be started at the last tolerated dose. The drug labeling has been updated to include dosing recommendations and retreatment safety and efficacy data. Bortezomib was previously approved for treatment of patients with multiple myeloma and for treatment of patients with mantle cell lymphoma who have received at least one prior therapy.
Supporting Study
Approval of retreatment was based on observation of durable responses in a single-arm phase II study in 130 adult patients who had achieved at least partial response on a prior bortezomib-containing regimen.1,2 Patients had received a median of two prior lines of therapy (range, 1–7). Patients with peripheral neuropathy or neuropathic pain of grade ≥ 2 were excluded from the study. Treatment was restarted at ≥ 6 months after prior bortezomib treatment at the last tolerated dose of 1.3 mg/m2 (n = 93) or ≤ 1.0 mg/m2 (n = 37) given intravenously on days 1, 4, 8, and 11 every 3 weeks for a maximum of eight cycles with or without dexamethasone. Overall, 83 patients received dexamethasone in cycle 1 and an additional 11 received it in later cycles.
European Group for Blood and Marrow Transplantation responses (49 partial and 1 complete) were observed in 50 patients (overall response rate = 38.5%, 95% confidence interval = 30.1%–47.4%). Median duration of response in responders was 6.5 months (range, 0.6–19.3 months).
How It Is Given
The recommended starting dose of bortezomib in multiple myeloma is 1.3 mg/m2. It can be given intravenously at a concentration of 1 mg/mL or subcutaneously at a concentration of 2.5 mg/mL.
Bortezomib retreatment can be started at the last tolerated dose and should be given twice weekly on days 1, 4, 8, and 11 every 3 weeks for a maximum of eight cycles. Consecutive doses should be separated by > 72 hours. Bortezomib can be administered as a single agent or in combination with dexamethasone.
Safety in Retreatment
The most commonly reported adverse events (incidence ≥ 20%) in clinical studies of bortezomib include nausea, diarrhea, thrombocytopenia, neutropenia, peripheral neuropathy, fatigue, neuralgia, anemia, leukopenia, constipation, vomiting, lymphopenia, rash, pyrexia, and anorexia.
The safety profile of bortezomib in the study supporting retreatment was consistent with the known safety profile of the drug in patients with relapsed multiple myeloma. No cumulative toxicities were observed with retreatment.
The most common adverse event of any grade was thrombocytopenia, which occurred in 52% of patients; grade ≥ 3 thrombocytopenia occurred in 24%. Peripheral neuropathy occurred in 28% of patients and was of grade ≥ 3 in 6%.
Serious adverse events occurred in 12.3% of patients, with the most common being thrombocytopenia (3.8%), diarrhea (2.3%), herpes zoster (1.5%), and pneumonia (1.5%). Adverse events led to discontinuation of treatment in 13%, with reasons including peripheral neuropathy (5%) and diarrhea (3%). Deaths considered to be drug-related occurred in two patients, one due to cerebrovascular accident and one due to sepsis, within 30 days of the last bortezomib dose.
Bortezomib carries warnings/precautions for peripheral neuropathy, cardiac toxicity, pulmonary toxicity, posterior reversible encephalopathy syndrome, gastrointestinal toxicity, thrombocytopenia and neutropenia, tumor lysis syndrome, hepatic toxicity, and embryo-fetal risk.
Report Adverse Events
Health-care professionals should report all serious adverse events suspected to be associated with the use of any medicine or device to FDA’s MedWatch Reporting System by completing a form online at www.fda.gov/medwatch/report.htm, by faxing (800-FDA-0178), by mailing the postage-paid address form provided online, or by telephone (800-FDA-1088). ■
References
1. VELCADE® (bortezomib) for injection prescribing information, Millennium Pharmaceuticals, Inc, August 2014. Available at www.velcade.com/files/pdfs/velcade_prescribing_information.pdf.
2. Petrucci MT, Giraldo P, Corradini P, et al: A prospective, international phase 2 study of bortezomib retreatment in patients with relapsed multiple myeloma. Br J Haematol 160:649-659, 2013.