Keratoacanthomas and cutaneous squamous cell carcinomas are frequently observed in patients receiving the RAF inhibitor vemurafenib (Zelboraf) for treatment of BRAF-mutated melanoma. As discussed by Lacouture and colleagues in a recent Journal of Clinical Oncology article, these effects appear to result from accelerated growth of lesions with HRAS mutations, and this accelerated growth appears to be inhibited by cotreatment with mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK, or MEK) inhibitors.1
Paradoxical Problem
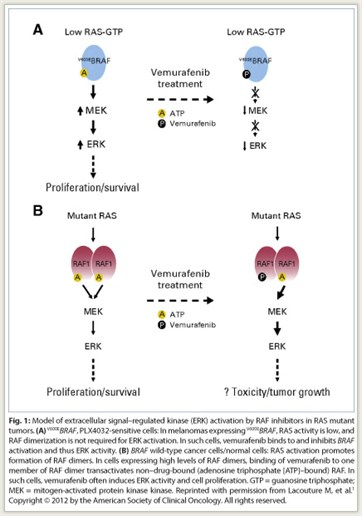 Vemurafenib is approved for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an FDA-approved test. Highly selective RAF inhibitors, such as vemurafenib and sorafenib (Nexavar), inhibit RAF activation of ERK only in tumors expressing mutant BRAF; paradoxically, these inhibitors activate this pathway in tumors with wild-type BRAF and in normal cells (Fig. 1). This signaling appears to account for the skin toxicity and cutaneous squamous cell carcinomas associated with vemurafenib treatment.
Vemurafenib is approved for the treatment of patients with unresectable or metastatic melanoma with the BRAF V600E mutation as detected by an FDA-approved test. Highly selective RAF inhibitors, such as vemurafenib and sorafenib (Nexavar), inhibit RAF activation of ERK only in tumors expressing mutant BRAF; paradoxically, these inhibitors activate this pathway in tumors with wild-type BRAF and in normal cells (Fig. 1). This signaling appears to account for the skin toxicity and cutaneous squamous cell carcinomas associated with vemurafenib treatment.
Approximately one-third of patients treated with vemurafenib develop a keratosis pilaris-like rash, and up to one-third develop keratoacanthomas or cutaneous squamous cell carcinomas. These adverse effects are not a major deterrent to use of vemurafenib in advanced melanoma; the keratoacanthomas and cutaneous squamous cell carcinomas are of low metastatic potential and are easily treated by such methods as surgical resection and cryotherapy. However, there is concern over the use of vemurafenib or other RAF inhibitors in earlier disease, as it remains unknown whether these agents might promote growth of tumors at other sites.
Recent Investigations
Two studies characterized mutations in the keratoacanthomas and cutaneous squamous cell carcinomas in patients receiving RAF inhibitors. As reported in the Journal of Clinical Oncology, Oberholzer and colleagues performed genomic analysis of 237 such lesions, including 19 from patients who had received RAF inhibitor therapy with either vemurafenib or sorafenib.2 They found that 21% of lesions from patients receiving RAF inhibitors had activating HRAS mutations compared with only 3% of lesions from patients not receiving these drugs.
Su and colleagues reported in The New England Journal of Medicine that 60% of 35 lesions from patients receiving vemurafenib harbored HRAS mutations, with the most prevalent being HRAS Q61L.3 Increased proliferation of HRAS Q61L–mutant cell lines exposed to vemurafenib was associated with MAPK-pathway signaling and activation of ERK-mediated transcription. These investigators then showed that a vemurafenib analog accelerated growth of lesions with HRAS mutations in a mouse model of HRAS Q61L–mediated skin carcinogenesis, but did not initiate or promote carcinogenesis. The growth promoted by the vemurafenib analog was blocked by concomitant treatment with a MEK inhibitor.
Thus, the emerging picture of vemurafenib-associated cutaneous squamous cell carcinoma is that induction of RAF signaling by the agent promotes the hyperproliferation of preexistent dormant RAS-mutant cancer cells—hence the concern that use of selective RAF inhibitors in patients with early cancer could be associated with risk of promoting growth of such dormant tumors in other sites.
It also appears that combining RAF inhibitors and MEK inhibitors may be a strategy for avoiding these effects of RAF inhibitors in the setting of mutated BRAF codon 600. RAF and MEK inhibitors both inhibit ERK pathway activation in tumors with mutations in BRAF codon 600. However, in contrast to RAF inhibitors, MEK inhibitors also inhibit this pathway when wild-type BRAF is present. Thus, use of such agents in combination may result in synergistic effects in BRAF-mutant tumors and reduce the toxicity associated with each. In a phase I trial of such a combination, the toxicity of the combination appeared to be less than that with either agent alone, and a randomized phase II trial of the combination is underway in patients with BRAF-mutant melanoma.4 ■
References
1. Lacouture ME, O’Reilly K, Rosen N, et al: Induction of cutaneous squamous cell carcinomas by RAF inhibitors: Cause for concern? J Clin Oncol 30:329-330, 2012.
2. Oberholzer PA, Kee D, Dziunycz P, et al: RAS mutations are associated with the development of cutaneous squamous cell tumors in patients treated with RAF inhibitors. J Clin Oncol 30:316-321, 2012.
3. Su F, Viros A, Milagre C, et al: RAS mutations in cutaneous squamous-cell carcinomas in patients treated with BRAF inhibitors. N Engl J Med 366:207-215, 2012.
4. Infante JR, Falchook GS, Lawrence DP, et al: Phase I/II study to assess safety, pharmacokinetics, and efficacy of the oral MEK 1/2 inhibitor GSK1120212 (GSK212) dosed in combination with the oral BRAF inhibitor GSK2118436 (GSK436). J Clin Oncol 29(suppl):526s, Abstract CRA8503, 2011.

 “The development of nonmelanoma skin cancers secondary to RAF inhibitor treatment has been recognized since 2005, having been seen initially with sorafenib (Nexavar),” noted Mario E. Lacouture, MD, of Memorial Sloan-Kettering Cancer Center. Commenting on recently published studies in this setting,...
“The development of nonmelanoma skin cancers secondary to RAF inhibitor treatment has been recognized since 2005, having been seen initially with sorafenib (Nexavar),” noted Mario E. Lacouture, MD, of Memorial Sloan-Kettering Cancer Center. Commenting on recently published studies in this setting,...